1As noted before, between acute exacerbations there may be limited symptoms associated with asthma. Even pulmonary function tests may be normal.
However, acute exacerbations results in many symptoms including wheezing, cough, and dyspnea.
In an acute asthma attack, wheezing will probably be most prominent, reflecting turbulent airflow through narrowed airways. Although wheezing often becomes more prominent with progressive airway constriction, it is also possible that when obstruction becomes sufficient, wheezing declines as there is insufficient airflow to create sounds. Cough associated with asthma can be nonproductive or associated with significant sputum.
Even in the absence of infection, sputum may be discolored, yellow, reflecting eosinophilic content and debris.
Obstructive severity, predicts the extent of dyspnea. With significant airflow obstruction, dyspnea may be very prominent with patients sitting up to ease breathing.
Chest tightness may be associated with asthmatic patients with dyspnea, reflecting symptoms that otherwise would suggest angina.
Severity of expiratory airflow obstruction is reflected in FEV1 as well as in maximum mid-expiratory flow rate. Spirometric data, as discussed earlier, helps to quantify the extent of pulmonary impairment and provide a baseline against which therapeutic management may be compared. Compare the two spirograms below:
|
|
|
1,2The figure on the left represents a normal spirographic volume-time relationships a normal spirogram, whereas the figure on the right represents spirographic volume-time relationships of a patient in bronchospasm. In the obstructive airway case (right) the FEV1 is < 80% of the vital capacity. Peak flow and maximum mid-expiratory flow (FEF25-75) are also attenuated in the obstructive disease example.
1 In terms of pulmonary function test results for individuals experiencing acute asthma, FEV1 is often < 35% of normal with a maximum mid-expiratory flow rate of < 20% of normal. Also, flow-volume spirometric loops show what is referred to as an "downward scooping" seen in the expiratory part of the loop.
|
|
1,2Severity of airway obstruction and pulmonary function test parameters
Mild (no symptoms): FEV1 as % of predicted = 65%-80%; FEF25%-75% as % of predicted = 60%-75%; PaO2 (estimated, mmHg) = >60; PaCO2 (estimated, mmHg)= < 40
Moderate: FEV1 as % of predicted = 50%-64%; FEF25%-75% as % of predicted = 45%-59%; PaO2 (estimated, mmHg) = >60; PaCO2 (estimated, mmHg)= < 45
Marked: FEV1 as % of predicted = 35%-49%; FEF25%-75% as % of predicted = 30%-44%; PaO2 (estimated, mmHg) = <60; PaCO2 (estimated, mmHg)= > 50
Severe (e.g. status asthmaticus): FEV1 as % of predicted < 35%; FEF25%-75% as % of predicted < 30%; PaO2 (estimated, mmHg) = <60; PaCO2 (estimated, mmHg)= > 50
1Asthma as a group of disorders
Allergen-induced
Exercise-induced
Nocturnal asthma (sleep-induced airway smooth muscle tone changes; reduced circulating catecholamine levels; reduced cough reflex)
Aspirin-induced
Aspirin along with many nonsteroidal anti-inflammatory drugs can induce bronchospasm in up to 20% of adult asthma patients.
Sensitive patients may exhibit airflow obstruction worsening and other symptoms within fifteen minutes to four hours following aspirin doses as little as 10 mg.
Aspirin-mediated inhibition of cyclooxygenase-promoted metabolism of arachidonic acid to prostaglandins which has the effect of increasing arachidonic acid conversion to leukotrienes, which are bronchoconstrictive, represents a possible mechanism of aspirin-induced bronchoconstriction.
About 5% of asthmatic patients are sensitive to certain food preservatives and antioxidants, some of which are present even in medications used to manage asthma.
1Occupational asthma
Occupational asthma which affects about 5% to 10% of the world population is probably the most common occupational lung disease.
About 15% of newly identified asthma cases appear related to occupational exposure (USA).
Chlorine and ammonia are probably the most common causes of occupational asthma that do not exhibit significant latency between exposure and effect.
By contrast, other occupational agents may be IgE-dependent. Such immune system dependency requires a longer time period before symptoms manifest.
In terms of the operating room environment, latex sensitivity may cause an increase in expiratory obstruction in sensitive individuals.
1Infectious asthma
This designation is for asthma (increased airway resistance) caused by acute inflammation due to pathogens, such as viruses, bacteria, or mycoplasma.
Bronchoconstriction due to this cause attenuates rapidly as the infection is treated
1More about Pharmacological Intervention
In recognition of the underlying inflammatory disease which characterizes asthma, administration of inhaled corticosteroids has become an important first-line of therapy in managing inflammatory component. Acute increases in bronchomotor tone are managed often to the use of beta 2 adrenergic agonists. Anticholinergic agents, particularly ipratropium are effective in reducing parasympathetic tone which in turn reduces bronchomotor tone.
Anti-inflammatory agents (corticosteroids & cromolyn):
Corticosteroids which reduce inflammation are used in a prophylactic context in asthma, given that their effects are not immediate. Corticosteroids which appear effective in controlling chronic asthma, reducing the likelihood of an acute attack, is preferably given by inhalation.
Oral administration would presumably increased the likelihood of systemic side effects.
The inhaled drug directly promotes an anti-inflammatory effect at the bronchial smooth musclel. Reduction in airway inflammation has the effect of reducing airway hyperreactivity although the period maximum benefit may be obtained only after the treatment has been in place for several months. Agents which are available for administration by inhalation include beclomethasone, triamcinolone, flunisolide, fluticasone, and budesonide
Pharmacokinetics: Most of an inhaled corticosteroids dose is swallowed (80%-90%). This drug and will be available systemically eventually passing to the liver. This leaves about 10% to 20% of the drug available for action at the bronchial level. This drug still has access to the systemic circulation, but drug's lipophilic character favors entry into the airway cells, their principal site of action, where the steroids can inhibit gene transcription for cytokines that promote airway inflammation.
Side-Effects:
Local and systemic side effects occur following corticosteroids inhalation.
Local effects include: hoarseness (dysphonia), pharyngitis, glossitis, oropharyngeal candidiasis.
Laryngeal muscle myopathy may cause dysphonia, which will be reversed following cessation of treatment.
Infection incidence is not increased by inhaled corticosteroids.
Systemic side effects are determined by the amount of systemic absorption.
Although corticosteroids may suppress the hypothalamic-pituitary- adrenal axis, inhaled corticosteroids amounts used in asthma management are not likely to have significant effect on pituitary-adrenal function. The inhaled corticosteroids do not appear to exert metabolic effects, alter bone metabolism, or interfere with growth.
Furthermore, parturients may be safely administered inhaled corticosteroids.
Cromolyn:
The classification of cromolyn as an anti-inflammatory agent is based on its apparent ability to inhibit chemical mediator release from mast cells, thus inhibiting inflammation. This agent is inhaled and is effective prophylaxsis, when used, for example, prior to expected exposure to a provocative activity or substance. For example, it should be used prior to exercise in patients known to have exercise-induced bronchospasm.
Leukotriene Inhibors: These agents are discussed in chapter 3.
1Beta adrenergic agonists:
As discussed earlier these agents bind to
![]() 2
adrenergic receptors and activate them. Activation of the second
messenger system results in an increase in cAMP production and
ultimately in relaxation of smooth muscle. These agents are helpful
in management of acute bronchospasm with albuterol perhaps being the
most commonly used agent.
2
adrenergic receptors and activate them. Activation of the second
messenger system results in an increase in cAMP production and
ultimately in relaxation of smooth muscle. These agents are helpful
in management of acute bronchospasm with albuterol perhaps being the
most commonly used agent.
Albuterol is administered by metered-dose
inhaler, using 2-3 deep inhalations, separated by 1-5 minutes.
This dosage may be repeated every 4-6 hours. Side effects are
minimized by this route of administration; however, some
systemic effects occur and are understood in terms of
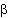 adrenergic receptor stimulation. Side effects include cardiac
arrhythmias, including tachycardia, as well as potassium
intracellular shifting.
adrenergic receptor stimulation. Side effects include cardiac
arrhythmias, including tachycardia, as well as potassium
intracellular shifting.
|
1Anticholinergic agents:
Individuals exhibit varying degrees of parasympathetic bronchiolar tone, which is bronchoconstrictive in effect. Accordingly, the effects of antimuscarinic agents will be proportional to the degree of underlying parasympathetic tone.
Metered-dose inhalation of ipratropium is effective in reducing parasympathetic-mediated bronchiolar smooth muscle tone.
Ipratropium is effective in management of bronchoconstriction in COPD patients; moreover it is helpful in management of asthma although the magnitude of the effect would be typically less than that observed with beta 2 adrenergic agonists.
Significant bronchodilator effect caused by ipratropium would usually be observed within 15-30 minutes of administration and the effect may persist at some level for 4-6 hours.
Ipratropium possesses a quaternary nitrogen and is therefore permanently positively charged. This condition of permanent positive charge explains the relatively limited systemic effects as a result of poor adsorption
Management of status asthmaticus
Definition: Status asthmaticus is a bronchospastic state that does not resolve during the initial, standard treatment, and bronchospasm is sufficiently severe such that a condition could be life-threatening.
In the emergence setting, effective treatment typically involves administration of β2 receptor agonist by inhaler.
For patients under 45 years of age, β2 agonist dosage may be repeated every 15-20 minutes up to 3-4 repetitions prior to significant systemic effects (hemodynamic). Concurrently, corticosteroids would be administered by IV.
Regimens for corticosteroid administration include either (a) cortisol 2mg/kg IV to be followed by 0.5 mg/kg/hour or (b) methylprednisolone at 60-125 mg IV every 6 hours.
Administration of supplemental oxygen may be required to ensure arterial oxygen saturation > 95%.
|
|
|
|
Severity of the acute exacerbation of asthma may be obtained through pulmonary function testing. For example, FEV1 at less than 25% of normal values suggest that the patient may develop hypercarbia. Hypercarbia (PaCO2 > 50 mm Hg) despite interventions noted above may indicate high risk for respiratory fatigue that may require management by tracheal intubation with mechanical ventilation.
With respect to mechanical ventilation: with high bronchoconstriction in this situation, there may be high peak airway pressure resistance that makes it difficult to deliver sufficient tidal volumes.
In the operating room setting, the anesthetic circuit, because of its volume, may contain excessive compressible volume, thus preventing adequate ventilation. Accordingly, low compressible volumes in anesthetic circuit would be desirable.
High gas flow rates would allow a shorter inspiratory time allowing adequate time for exhalation and a lower positive end-expiratory pressure (auto-PEEP). In the severe asthmatic, the expiratory phase may have to be lengthened to allow adequate time for exhalation and to prevent auto-PEEP. An alternative to this approach is to allow hypercarbia to persist, thus avoiding barotrauma.
Minimal or no symptoms are noted when FEV1 reaches at least 50% normal. When this condition is reached, reduced bronchodilator treatment may be warranted.
Under some circumstances patients will not respond to these interventions, perhaps because the airflow obstruction is mainly due to airway inflammation, intralumenal secretion, and edema.
![]() Mucus-plugging
can be a serious problem with patients had risk for asphyxia.
Mucus-plugging
can be a serious problem with patients had risk for asphyxia.
The above, aggressive pharmacological intervention may be insufficient to reverse bronchoconstriction states and bronchodilatation may be attempted using general anesthetics.
Effective therapies using general anesthetics may include halothane, enflurane, or isoflurane; however, this type of intervention is reserved for the special cases in which other aggressive bronchodilator-steroid protocols have proven ineffective.
|
4Que, JC and Lusaya, VO Sevoflurane Induction for Emergency Cesarean Section in a Parturient in Status Asthmaticus, Anesthesiology, 1999; 90: 1475-1476.
|
1Anesthesia Management in the Asthmatic Patient
The central point of preoperative assessment is to develop in anesthesia plan which either prevents or facilitates the management of airflow obstruction. The clinical history is important in assessing the primary characteristics of a particular patients asthma. The characteristics include:
|
Age of onset |
Identified triggering events |
History of hospitalization for asthma |
Identified allergies |
|
Cough
|
Changes in color and characteristics of sputum |
Previous anesthesia history |
Current medications |
Auscultation should reveal whether or not the patient is experiencing an acute asthma exacerbation (presence or absence of wheezing).
An indirect preoperative assessment of disease status may be derived from eosinophil counts which appear to parallel the extent of airway inflammation. Recall here that asthma is fundamentally a chronic inflammatory disease.
In patients with known bronchial asthma who are going to undergo substantial but elective surgery, pulmonary function tests which compare FEV1 before and after bronchodilator treatment may be appropriate. Preoperative interventions that may improve reversible elements of asthma include adequate systemic hydration, antibiotics, bronchodilator treatment, and chest physiotherapy.
Any change in the disease process may be revealed by comparative chest radiography.
Uncertainty concerning ventilation adequacy or adequacy of arterial oxygenation would mandate arterial blood gas analysis prior to elective surgery.
There may be no specific preferred drug or combination of drugs that has been shown definitively preferable for preoperative medication. For example, opioids used in preanesthetic dosages are not thought to produce reflex or direct bronchoconstriction or to stimulate vasoactive substance release from mast cells.
On the other hand, ventilatory depression mediated by opioids should be considered. Antiholinergic agents may be useful; however, these agents increase secretion viscosity making it perhaps more difficult to maintain a clear airway.
Airway resistance which theoretically would be lessened by the antimuscarinic agents is unlikely to obtain with the typical preanesthetic anticholinergic drug dosage (intramuscular).
H2-receptor blocker administration to asthmatic patients appears questionable because bronchodilation is mediated by H2 receptors. Bronchodilator drugs routinesly used to manage asthma should be continued to the time of anesthesia induction.
An example would be cromolyn which does not interact adversely with anesthesia drugs, allowing cromolyn use to be continued during the immediate preoperative timeframe.
Exogenous corticosteroid supplementation might be appropriate before major surgery if previous corticosteroid administration had been part of the asthma control regimen. The rationale here is that chronic corticosteroid administration may have suppressed the hypothalamic-pituitary-axis. This type of suppression is considered unlikely. There is inadequate data to suggest that all patients with asthma should receive preoperative corticosteroids -- there is a relatively low likelihood of perioperative morbidity in any case, particularly in patients with inactive asthma as defined by preoperative assessment.
If preoperative FEV1 is determined to be < about 80%, oral corticosteroids may be useful.
Finally, since aspirin or NSAIDs can induce asthma, these agents must be used cautiously for management of postoperative pain.
1Induction and Anesthesia Maintenance
A principal objective in anesthesia induction and maintenance for asthma patients is to reduce the likelihood of airway reflex activation that might cause bronchoconstriction. The presumption is that the asthmatic patient will have hyperreactivity airways. Normal stimuli that would be of limited interoperative consequence in a normal patient may induce potentially fatal bronchoconstrictive responses in the asthmatic patient.
1Regional Anesthesia
If the operative location is superficial or on the extremities, regional anesthesia may be a good option. The reason is that avoiding airway manipulation (associated with tracheal intubation) reduces the likelihood of direct provocation of bronchoconstrictive responses. Bronchospasm theoretically occurring as a result of high sensory level anesthesia and sympathetic blockade does not appear to be the clinical issue. General anesthesia may be required if, for some reason, regional anesthesia appears ineffective.
1General Anesthesia.
Anesthesia induction is often initiated with IV administration using short-acting agents such as thiopental, propofol, or etomidate.
Wheezing appears more likely in the asthmatic patient induced with thiopental compared to propofol. Propofol administration appears to be associated with reduced respiratory resistance following tracheal intubation in healthy patients, relative to etomidate or thiopental use.
Propofol's bronchodilating effect is probably neural in nature.
Propofol therefore may be a reasonable inducing agent in patients who are asthmatic but are hemodynamically stable, particularly as tracheal intubation or upper airway stimulation is likely to follow.
Another option might be ketamine which has sympathomimetic effects which could promote smooth muscle relaxation and reduce airway resistance particularly in wheezing asthmatic patients .
Thiopental itself probably does not induce bronchospasm but airway stimulation which could cause reflex-mediated bronchospasm is unlikely to be attenuated by thiopental.
Asthma-inducing chemicals, particularly preservatives, may be a concern since metabisulfites, found in a generic propofol formulation, may cause bronchospasm.
Following induction with an IV agents, a volatile anesthetic is likely to be used to establish appropriate anesthetic depth. The depth of anesthesia is likely sufficient to suppress hyperreactivity airway reflexes such that tracheal intubation can proceed without inducing bronchospasm.
As a corollary, inadequate anesthesia depth may itself be a cause of bronchospasm induced by airway manipulation.
Comparable doses of volatile anesthetics may not all exhibit the same bronchodilator properties.
For example, in an animal model, 1.7 MAC halothane was more effective than isoflurane, when bronchospasm was induced by histamine.
Reduced coughing due to the absence of a pungent odor observed with sevoflurane and halothane may be important since coughing itself is a bronchospasm trigger.
Halothane is not probably ideal since it does sensitize the myocardium in circulating catecholamines and as such a precipitate arrhythmias.
In this sense halothane would be a pro-arrhythmic volatile agent.
Suppression of airway reflexes by volatile anesthetics will blunt the bronchoconstrictive responses; moreover, airway reflex suppression might also be obtained using lidocaine (1.5 mg/kg IV) administered 1-3 minutes prior to direct laryngoscopy and tracheal intubation (bronchoconstriction by reflex mechanism appears suppressed).
Intratrachial lidocaine administered just prior to endotracheal tube of placement may be beneficial; however application of lidocaine onto the hyperreactivity airway surface may itself induce bronchospasm.
Generally, if adequate anesthesia is present, intratrachial lidocaine probably does not result in bronchospasm.
In terms of reducing airway responses to tracheal intubation in asthmatic patients, inhaled albuterol may be more effective than intravenous lidocaine (although intravenous lidocaine does cause bronchodilatation)
Following tracheal intubation, one challenge is to discriminate between light anesthesia and bronchospasm, both causing reduced pulmonary compliance.
Neuromuscular blocking drug administration would tend to relieve ventilatory resistance due to light anesthesia but would be expected to have no effect on ventilatory resistance due to bronchospasm.
1Skeletal Muscle Relaxation:
Nondepolarizing muscle relaxants are used to provide muscle relaxation during anesthesia maintenance.
Most agents have about the same likelihood of inducing bronchospasm.
Increased airway resistance following succinylcholine use, despite possible succinylcholine-mediated histamine release, has not been observed.
Reversal or antagonism of nondepolarizing agents with acetylcholinesterase inhibitors poses at least a theoretical concern that the anticholinesterase agents would increase acetylcholine concentration thereby promoting bronchospasm.
However, this effect is not observed clinically, with the possible explanation that simultaneous administration of antimuscarinic agents may prevent bronchospasm occurrence
1Other issues: the endotracheal tube in the asthmatic patient should be removed when the depth of anesthesia is still adequate to suppress airway reflexes. If it is considered inappropriate to extubate before the patient is awake, effort should be made to minimize airway stimulation due to the tracheal tube removal. In this regard, IV lidocaine infusion (1-3 mg/kg/hour) may be helpful.
Intraoperative bronchospasm is usually not associated with acute asthmatic attack; therefore, it is appropriate to first consider wheezing due to mechanical breathing circuit obstructions or obstruction in the patients airway, including the endotracheal tube.
Fiber-optic bronchoscopy may be definitive in ruling out mechanical obstruction causing bronchospasm.
If the bronchospasm cannot be associated with the delivery system and in the absence of an acute asthma exacerbation, management may include adjusting the anesthesia depth or initiating skeletal muscle paralysis.
Bronchospasm due to an actual asthma attack will respond to deepening the anesthesia with the volatile agent; however, the resolution is not likely as a result of skeletal muscle paralysis due to muscle relaxants administration.
If bronchospasm continues
following adjustment of anesthesia depth, ![]() adrenergic agonists agents may be used. Therefore, albuterol
could be provided to the patient by applying the metered-dose
inhaler to the anesthesia delivery system by the T-connector.
The metered dose inhaler would deliver about 90 micrograms of
albuterol per actuation. Despite such intervention, if
bronchospasm continues, addition of corticosteroids could
be considered even recognizing that a corticosteroid therapeutic
effect may be delayed 3-4 hours.
adrenergic agonists agents may be used. Therefore, albuterol
could be provided to the patient by applying the metered-dose
inhaler to the anesthesia delivery system by the T-connector.
The metered dose inhaler would deliver about 90 micrograms of
albuterol per actuation. Despite such intervention, if
bronchospasm continues, addition of corticosteroids could
be considered even recognizing that a corticosteroid therapeutic
effect may be delayed 3-4 hours.
1Differential Diagnosis of Bronchospasm/Wheezing Encountered During Surgery (more likely to less likely) [Table 14-3, from ref. 1]
Mechanical obstruction of the endotrachial tube due to:
kinking
secretions
tracheal tube cuff overinflation
Anesthesia depth: TOO LIGHT
active effort on expiration
reduced FRC
"Endobronchial intubation
Pulmonary aspiration
Pulmonary embolus
Pneumothorax
Acute asthma attack"
1Emergency surgery setting:
In this circumstance, competing interest include airway protection of patients at risk for aspiration on one hand and risk of triggering bronchospasm due to light anesthesia. Awake endotrachial intubation can cause stimulations of tracheal and laryngeal reflexes and thereby promote bronchospasm. Bronchodilator treatment plans may be unattainable due to time pressure. Regional anesthesia may be the best option if circumstances permit.
1Stoelting, RK and Dierdorf, SF, "Asthma" in Anesthesia and Co-Existing Disease, 4th edition, Chapter 14, pp 194-204, Churchill-Livingstone, Philadelphia, 2002
2Kingston, HGG, Hirshman, CA Perioperative management of the patient with asthma. Anesth Analg 1984; 63: 844-55. (second sourced from reference 1)
3Roisen, MF, "Anesthetic Implications of Concurrent Diseases" in Anesthesia, 5th edition, (Miller, R.D, editor), Chapter 25, pp 996-997, Churchill-Livingstone, Philadephia 2000
4Que, JC and Lusaya, VO Sevoflurane Induction for Emergency Cesarean Section in a Parturient in Status Asthmaticus, Anesthesiology, 1999; 90: 1475-1476.
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |