|
|
|
Anesthesia Pharmacology Chapter 1: General Principles: Overview and Introduction
![]()
Barbiturates
| Pentobarbital sodium (Nembutal) | 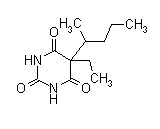 |
|
| Thiopental sodium (Pentothal) | 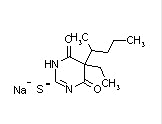 |
|
| Butabarbital sodium (Butisol) | 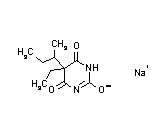 |
|
| Thiamylal | 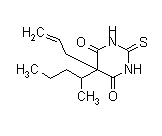 |
|
| Amobarbital sodium | 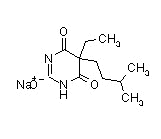 |
|
Metabolism:
Many enzymes exhibit stereoselectivity, a preference for one or the other enantiomeric form:
In the case of drug-metabolizing enzymes, stereoselectivity may result in a difference in the duration of action of one enantiomer compared to the other.
Structure Activity Relationships:
Understanding the relationship between drug structures and biological activities forms the basis of rational drug design.
Computer-enhanced molecular modeling and information concerning three-dimensional receptor structure may combine to improve the effectiveness of rational drug design approaches.
Katzung, B. G. "Basic Principles: Introduction" in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, p.1-4
Stoelting, R.K., "Pharmacokinetics and Pharmacodynamics of Injected and Inhaled Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 1-17
3Dolin, S. J. "Drugs and pharmacology" in Total Intravenous Anesthesia, pp. 13-35 (Nicholas L. Padfield, ed), Butterworth Heinemann, Oxford, 2000