|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiviral Drugs
Acyclovir and Related Drugs continued:
The broadest application of acyclovir is in the treatment of genital herpes simplex virus infections.2
IV or oral acyclovir as well as oral valacyclovir reduce symptom duration, and viral shedding, while facilitating healing in primary genital HSV infection.2
Both of these drugs, valacyclovir and acyclovir, have limited effect on recurrent genital HSV infections.
In considering either primary or recurrent genital HSV infection, neither drug changes the likelihood of subsequent recurrences, suggesting that acyclovir administration does not eliminate latent infection.
However, continual acyclovir oral administration (up to six years) or continual valacyclovir administration (greater than or equal to one year) decreases recurrence frequency during therapy; however, upon drug discontinuation, HSV lesions recur.
For patients with AIDS, chronic or episodic acyclovir administration promotes emergence of HSV and VZV resistant strains to acyclovir action.
![]() Clinical
failure results.2
Clinical
failure results.2
In this circumstance, the emergent isolate is likely deficient in virus-induced thymidine kinase which catalyzes initial phosphorylation step in activation of the pro-drug acyclovir.
![]() Patients
resistant to acyclovir with HSV or VZV infection often respond to
foscarnet.2
Patients
resistant to acyclovir with HSV or VZV infection often respond to
foscarnet.2
|
|
 |
|
|
Topical acyclovir has limited clinical indications; however, this formulation may be beneficial in primary genital HSV infections and in mucocutaneous herpes simplex viral infections in immunocompromised individuals.
Varicella-Zoster Virus (VZV) Infection
![]() Oral
acyclovir is therapeutically efficacious in treating varicella-zoster
viral infections (VZV) in both children and adults.1
Oral
acyclovir is therapeutically efficacious in treating varicella-zoster
viral infections (VZV) in both children and adults.1
Acyclovir in children decreases fever and new lesion formation by about 1 day.
Although acyclovir routine use in "uncomplicated" VZV infections is not recommended, the drug may be considered in individuals with:1
Risk of moderate-to-severe illness e.g. individuals greater than 12 years of age,
Individuals who would represent secondary household cases
Individuals with chronic pulmonary disorders or chronic cutaneous disorders
Individuals receiving corticosteroids or long-term salicylate treatment.
In adults treated within a day, oral acyclovir decreases time to lesion cresting by about two days.1
The drug also diminishes the maximum lesion number by about 50% and shortens fever duration.
IV acyclovir is likely effective in treating varicella-zoster pneumonia or encephalitis in previously healthy adults.1
Furthermore, as a preventive measure, oral acyclovir administered between one and two weeks following exposure may decrease risk of varicella infection.
For older adults with localized herpes zoster, orally acyclovir decreases pain and healing times when treatment is started within three days of rash onset.
Decreased ocular complications, especially keratitis and anterior uveitis due to zoster ophthalmicus, is associated with treatment.
Extended, concurrent administration of acyclovir and prednisone for three weeks accelerated zoster healing while improving quality-of-life measures when compared with each treatment regimen alone.1
Valacyclovir may provide quicker relief from zoster-associated pain compared to acyclovir in older adults (older than 50 years of age) with zoster.
Intravenous acyclovir administered in immunocompromised patients with herpes zoster reduces:1
Length of hospitalization
Viral shedding
Healing time and
Risks of cutaneous dissemination and visceral complications.
For immunosuppressed children with their varicella, IV acyclovir decreases healing time and reduces visceral complication risk.1
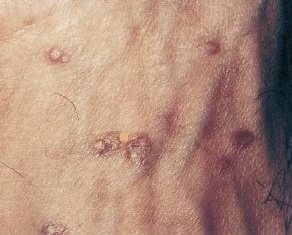
![]() Acyclovir-resistant VZV isolates occasionally have been identified in
HIV-infected children and adults manifesting chronic hyperkeratotic or
verrucous lesions and occasionally meningioradiculitis.1
Acyclovir-resistant VZV isolates occasionally have been identified in
HIV-infected children and adults manifesting chronic hyperkeratotic or
verrucous lesions and occasionally meningioradiculitis.1
VZV is likely the sole pathogenic agent in chronic verrucous (wart-like) lesions.8,9
 |
|
![]() For
acyclovir-resistant VZV disease, IV foscarnet may be effective.
For
acyclovir-resistant VZV disease, IV foscarnet may be effective.
|
Ganciclovir (Cytovene, Cymevene)
|
|
|
|
 |
![]() Ganciclovir (Cytovene, Cymevene) is an effective drug for
cytomegalovirus (CMV) prophylaxis in immunocompromised individuals.1
Ganciclovir (Cytovene, Cymevene) is an effective drug for
cytomegalovirus (CMV) prophylaxis in immunocompromised individuals.1
Acyclovir is not effective in established CMV infections.1
High-dose IV acyclovir and CMV-seropositive bone marrow transplant recipients reduces risk (about 50% reduction) of CMV disease.
When combined with long-term oral acyclovir, survival is enhanced. Following engraftment, valacyclovir is likely equally effective compared to IV ganciclovir prophylaxis in this patient subset.
Oral acyclovir or valacyclovir at high dose suppression for three months seems to decrease CMV disease risk in some cases.
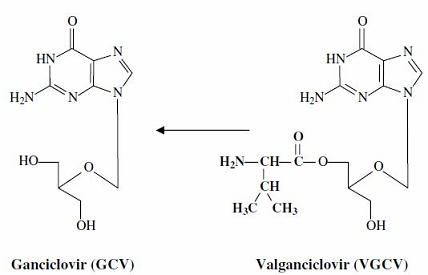
However, oral valganciclovir appears preferred for mismatched graft recipients.
Valganciclovir is the valine ester of ganciclovir, which has limited oral bioavailability.
Valganciclovir is later converted by valACVase to release the parent drug, ganciclovir.
 |
|
High-dose valacyclovir, compared with acyclovir, decreases CMV disease in advanced HIV infection; however, such administration is associated with higher toxicity and perhaps shorter survival.1
Oral acyclovir along with systemic corticosteroids may be helpful in treating Bell's palsy.1
|
|
|
|
Famciclovir (Famvir and generic)
|
|
 |
 |
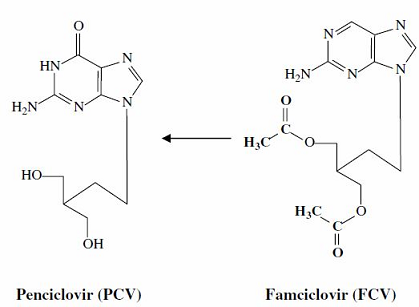
As noted below, famciclovir (Famvir) is a prodrug which is, upon deacetylation and hepatic oxidation (oxidation in the liver), converted to penciclovir (Denavir).3
Famcyclovir via penciclovir exhibits antiviral activity (in vitro) against:3
Herpes simplex virus (HSV-1; HSV-2)
Varicella-zoster virus (VZV)
Epstein-Barr virus (EBV)
Hepatitis B virus (HBV).
Antiviral activation requires an initial phosphorylation step as described previously for acyclovir.3
This step is catalyzed by a viral thymidine kinase found in infected cells.
![]() Additional
phosphorylation steps, catalyzed by host cell kinases, add two
additional phosphate groups leading to the ultimate active drug form,
penciclovir triphosphate.
Additional
phosphorylation steps, catalyzed by host cell kinases, add two
additional phosphate groups leading to the ultimate active drug form,
penciclovir triphosphate.
This agent inhibits viral DNA polymerase, thus diminishing viral replication.3
As described in more detail in the section on antiviral drug resistance, the most frequently encountered resistant HSV mutant isolates exhibit viral thymidine-kinase deficiencies.
Accordingly, cross-resistance between acyclovir and famciclovir (penciclovir) are expected.
Oral famciclovir is efficacious both for initial and recurrent management of genital herpes as well as for chronic genital herpes suppression.3
Also, the drug may be used for "cold sores" as well as for treatment of acute zoster (VZV).3
 |
|
|
|
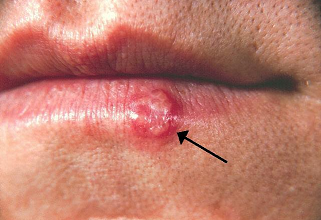
Penciclovir (Denavir) applied as a 1% cream, reduces both symptoms and signs of herpes labialis (a.k.a cold sore, fever blister, etc) in immunocompetent patients.
This finding was based on a randomized, placebo-controlled, double-blind, patient-initiated, clinical trial.13
For the study, patients were prospectively provided study medication with the treatment being initiated by the patient within 1 hour of the first sign or symptom of herpes simplex labialis (cold sore) recurrence.
About 2200 immunocompetent individuals were enrolled in the study and given study medication with about 1570 patients initiating application of topical 1% penciclovir cream for recurrence.
Vehicle control cream represented placebo.
If recurrence occurred, topical 1% penciclovir cream or vehicle control cream was applied every two waking hours for 4 consecutive days.
The primary efficacy variable was lesion healing; however, secondary endpoints involved time to loss of lesion pain and time to cessation of viral shedding.
Results indicated that healing of typical lesions, such as ulcers, vesicles and/or crusts occurred about 0.7 day faster in the penciclovir-treated patient group.13
![]() Penciclovir
cream is the first treatment that clearly demonstrated in effect on the
course of recurrent herpes labialis in immunocompetent patients.
Penciclovir
cream is the first treatment that clearly demonstrated in effect on the
course of recurrent herpes labialis in immunocompetent patients.
Efficacy was noted in both clinical and laboratory measures of disease.13
 |
|
The extent of the reduction is from a half to one day. This indication has been approved by the FDA.
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |