|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiretroviral Drugs Used in Treating HIV Infection
 |
|
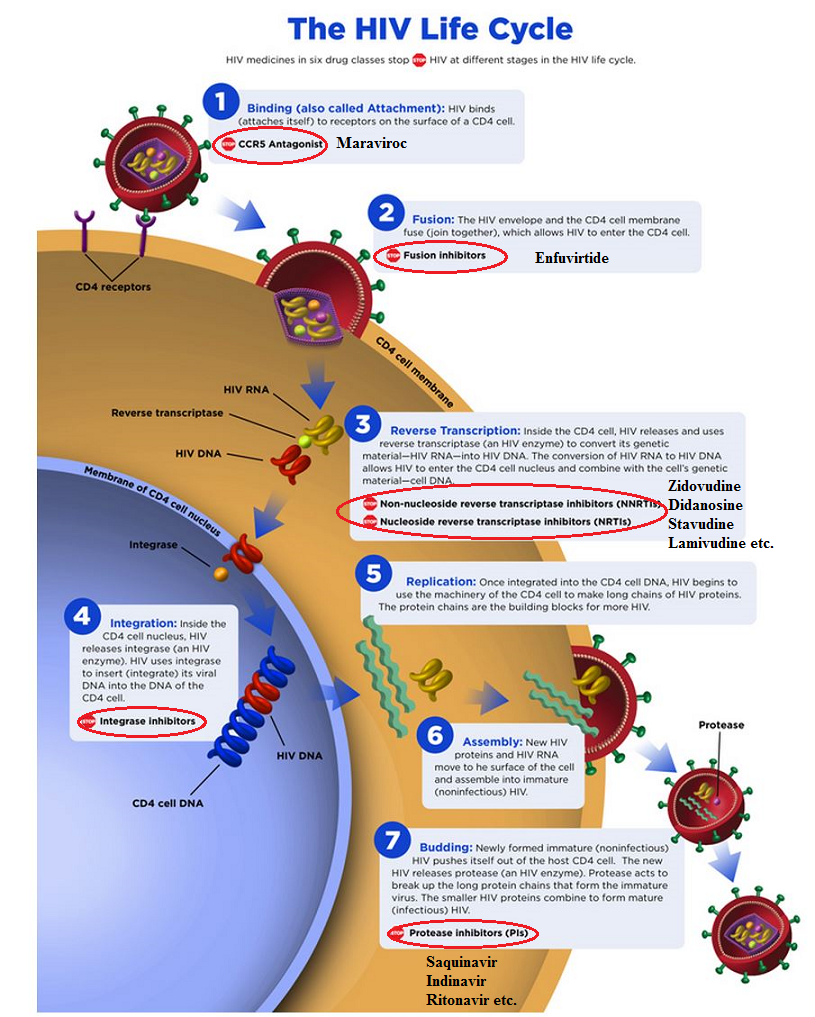
![]() There are four
classes of antiretroviral agents targeting HIV-specific enzymes.7
There are four
classes of antiretroviral agents targeting HIV-specific enzymes.7
These classes include:7
Nucleoside and nucleotide reverse-transcriptase inhibitors
Nonnucleoside reverse-transcriptase inhibitors
Protease inhibitors
Integrase inhibitors
Another class of anti-HIV drugs is are HIV cell entry inhibitors or CCR5 antagonists.
Here a specific, nonenzymatic protein is the pharmacological target.7
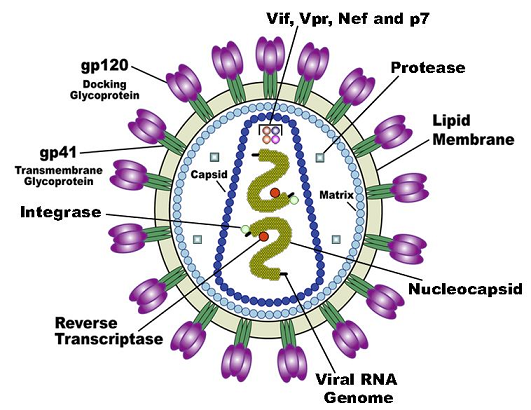
By review, the HIV virus targets cells based on the presence of the CD4 receptor.1
 |
|
The targeting is regulated by the viral envelope protein gp160 (env).
The CD4 receptor is present on both lymphocytes and macrophages.
HIV entry into host cells is also dependent on a chemokine receptor, either CCR5 or CXCR4.
CCR5 is associated with macrophage-type target cells.
Of the two chemokines, the CCR5 coreceptor is dominant although as the disease advances a shift from CCR5 to CXCR4 dependency has been observed.
![]() Also,
increased affinity of HIV-1 for the CXCR4 coreceptor allows T
lymphocyte infection.
Also,
increased affinity of HIV-1 for the CXCR4 coreceptor allows T
lymphocyte infection.
The transition between CCR5 to CXCR4 dependency is also correlated with an increased rate of loss of CD4+ helper T cells along with increasing likelihood of immunosuppression.1
The gp41 domain of env regulates virus-lipid bilayer fusion with the host cell lipid bilayer (membrane).1
 |
|
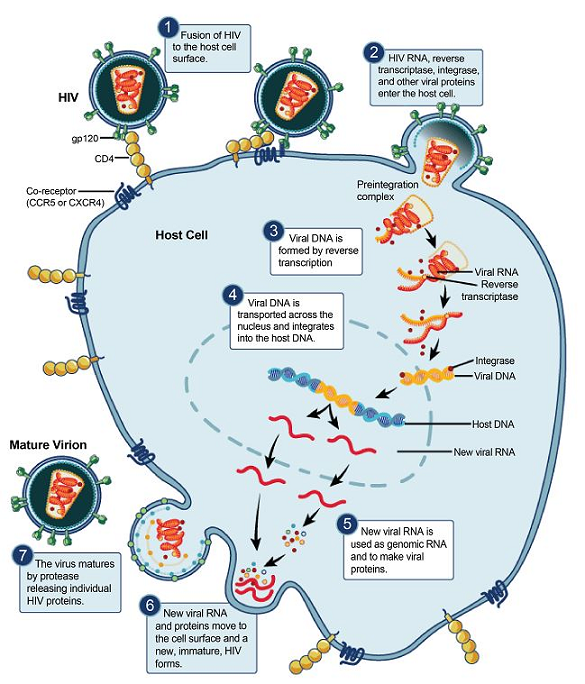
After fusion, viral RNA enters the cytoplasm and undergoes replication to a transient RNA-DNA duplex.
The initial RNA is degraded by RNase H, permitting synthesis of a full-length double-stranded DNA "copy" of the viral genome.
The error prone characteristic of the HIV reverse transcriptase enzyme results in HIV virus mutations.
![]() Mutations
occur in about three bases per full-length (9300-base-pair)
replication.
Mutations
occur in about three bases per full-length (9300-base-pair)
replication.
Following cytoplasmic-localized reverse transcription, viral DNA is translocated to the nucleus and integrated into a host chromosome by an HIV-viral integrase enzyme.
![]() This
viral integrase enzyme is a pharmacological anti-HIV target site.1
This
viral integrase enzyme is a pharmacological anti-HIV target site.1
More information about HIV viral-host cell integration may be found here.
After host cell genomic integration, the viral genome may remain latent i.e. not directing RNA or protein synthesis yet the viral genome is replicated as the cell divides.1
Cells containing the viral genome as part of its own genome may be activated and at that time viral RNA and proteins would be synthesized.
Genomic viral RNA is housed in a nucleocapsid with envelope and structural proteins concentrated at the cell surface, localized in cholesterol-rich "lipid rafts.".
Nucleocapsid cores concentrate at these sites and bud across the cell membrane, thus creating new envelope HIV virions with two complete single-stranded RNA genomes.
When these virions infect new cell, reverse transcriptase may initiate a new cycle of viral replication.1
 |
|
|
|
|
HIV infection due to sexual activity may be initiated by one or only a few infectious virions.1
After this event, a short, rapid replication phase occurs in the 2-4 week time frame.1
During this brief period ≥ 109 cells become infected.
This peak of infectious activity is accompanied by a temporary reduction in peripheral CD4+ (helper) T lymphocytes.
New host immune response combined with HIV-target cell depletion leads to an approximate steady-state level of infectious HIV virions.
This level is mirrored by plasma HIV RNA concentration, also reflecting "viral load".
This apparently relatively constant level of HIV virus activity results from a number of factors such as the host immune response, the pathogenicity of the particular HIV viral strain and defines a "set point".
This set point can be thought of as a balance between virion replication which increases the viral burden and clearance which reduces it.1
The early burst of activity is associated with acute symptoms, significant viremia and large numbers of infected target cells (CD4+ T cells) in blood.13
The early antiviral immune response involves antibodies and cytotoxic T cells and reduces circulating virus by at least a factor of 100, initiating the clinically latent phase characterized by low, yet constant presence of HIV virions and infected cells in circulation.
![]() During
this latent phase the number of CD4+ T cells gradually decline.
During
this latent phase the number of CD4+ T cells gradually decline.
![]() Clinical
latency may extend for about 10 years.
Clinical
latency may extend for about 10 years.
![]() Eventually,
the number of CD4 + T cells is reduced to extremely low values
coinciding with the development of acquired immunodeficiency
syndrome (AIDS).
Eventually,
the number of CD4 + T cells is reduced to extremely low values
coinciding with the development of acquired immunodeficiency
syndrome (AIDS).
The development of AIDS is also associated with rapidly increasing viral activity and increasing circulating infected cells.13
|
|
|
As noted earlier, most individuals progress to clinical AIDS in about a decade patients subsequent to sexual acquisition of CCR5-tropic HIV-1 disease (without antiretroviral treatment) .1
However, a subset of patients, described as "long-term nonprogressors" have been identified as having HIV infection for over 20 years without exhibiting immunosuppression or notable reduction in CD4 T cell counts.1,14
Long-term nonprogression may occur as a consequence of an interplay of individual immunogenetics and immune responses.1,14
|
|
|
Some long-term nonprogressors (probably a small subset) exhibit mutations of the CCR5 coreceptors; these mutations reduce HIV infectivity.14,15
Other factors may include different mitochondrial DNA haplotypes (group of genes inherited from one parent) which influences rates of HIV-1 disease progression.16
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |