|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
Antiretroviral Drugs Used in Treating HIV Infection
→Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
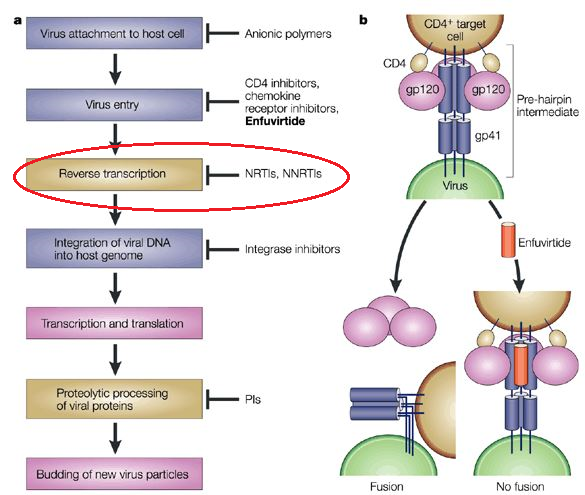 |
|
|
|
|
|
Zidovudine (AZT) |
Zidovudine (AZT) is a thymidine analogue suitable for oral administration. Usually, zidovudine is formulated along with lamivudine for daily dosing (2X).6
|
Thymidine |
|
Zidovudine (AZT) |
Dosage adjustment for zidovudine may be required for those patients with sufficient renal impairment requiring hemodialysis, peritoneal dialysis or continuous venovenous hemofiltration.
Zidovudine has an oral bioavailability of about 65% as a result of first pass (phase II) hepatic glucuronidation.
|
Zidovudine does not induce the microsomal cytochrome P450 drug metabolizing system and is not a substrate for that system either.
![]() Zidovudine
should not be co-administered with stavudine a.k.a. d4T as a result of
in vivo (and in vitro) antagonism..6
Zidovudine
should not be co-administered with stavudine a.k.a. d4T as a result of
in vivo (and in vitro) antagonism..6
The assessment of this antagonism was based on a randomized clinical study which involved adding stavudine (d4T) or didanosine (DDI) to HIV-infected individuals receiving zidovudine.9
Since both zidovudine and stavudine require thymidine kinase-mediated phosphorylation for activation, the parent compound are "prodrugs".
Furthermore zidovudine exhibits a >100-fold affinity for thymidine kinase compared to stavudine.
This characteristic was reflected in measurements of intracellular stavudine-triphosphate.
![]() In
absence of zidovudine, intracellular stavudine triphosphate
levels were about 65 fmol/106 cells (stavudine
alone).
In
absence of zidovudine, intracellular stavudine triphosphate
levels were about 65 fmol/106 cells (stavudine
alone).
By contrast, levels of stavudine triphosphate levels in patients also receiving zidovudine were about 10 fmol/106 cells or about 6-fold less.
These results, albeit in a small clinical study, were consistent with findings in the in vitro setting.9
Combination of zidovudine and stavudine represented a theoretical risk, since both drugs depend on intracellular phosphorylation by cellular thymidine kinase.4
The phosphorylation step is important because zidovudine is sequentially converted to 5'-mono-, di-, and triphosphates by thymidine kinase.
![]() Zidovudine
so phosphorylated is incorporated into proviral DNA, important
because viral reverse transcriptase uses
zidovudine-triphosphate as a substrate.
Zidovudine
so phosphorylated is incorporated into proviral DNA, important
because viral reverse transcriptase uses
zidovudine-triphosphate as a substrate.
The tri-phosphorylated form prevents normal 5', 3'-phosphodiester bonding, thus resulting in DNA chain elongation termination due to the presence of the azido group in zidovudine (AZT).4
Chain termination occurs because zidovudine 5'-triphosphate which is incorporated into nascent DNA lacks a 3'-hydroxyl group.1
The first phosphorylation step of zidovudine, yielding the monophosphate, results in a form that competitively inhibits cellular thymidylate kinase.1
This competitive inhibition reduces quantities of intracellular thymidine triphosphate.
Zidovudine 5'-triphosphate slightly inhibits cellular DNA polymerase-α; however, this compound is a stronger inhibitor of mitochondrial polymerase-γ.1
Conversion of zidovudine 5' monophosphate to diphosphate is relatively slow, resulting in monophosphate accumulation in cells.
Elevated concentrations of intracellular monophosphate acts as a reservoir and as a result, elevation in plasma zidovudine may not proportionally increase intracellular zidovudine triphosphate.1
|
|
|
Thymidine |
|
Zidovudine (AZT) |
Mutations and Zidovudine Resistance
Clinical resistance to zidovudine anti-retroviral activity is due to mutations in the viral reverse transcriptase enzyme.1
|
|
|
These mutations have been localized to specific codons.11
At codon 41 the amino acid leucine is substituted for the normally occurring wild-type amino acid methionine.
At codon 67 the amino acid asparagine is substituted for the normally occurring wild-type amino acid aspartate.
At codon 70 the amino acid arginine is substituted for the normally occurring wild-type amino acid lysine.
At codon 210 the amino acid tryptophan is substituted for the normally occurring wild-type amino acid leucine
At codon 215 either the amino acid tyrosine or phenylalanine is substituted for the normally occurring wild-type amino acid threonine .
At codon 219 either the amino acid glutamine or glutamate is substituted for the normally occurring wild-type amino acid lysine.
![]() These
mutations have been described as "thymidine analogue mutations"
or TAMs since they cause cross-resistance to other thymidine
analogues including stavudine.1
These
mutations have been described as "thymidine analogue mutations"
or TAMs since they cause cross-resistance to other thymidine
analogues including stavudine.1
Two groups of resistance mutations frequently occur.1
For example, mutations at codons 41, 210, and 215 is correlated with significant zidovudine resistance and cross-resistance to tenofovir and abacavir.
Reduced levels of resistance and cross-resistance are associated with mutations at codons 67, 70 and 219.
The consequence of thymidine analogue mutations to zidovudine and stavudine is one that enhances excision of incorporated nucleotide anabolites by means of pyrophosphorolysis.1
Pyrophosphorolysis is the reverse reaction of nucleotide polymerization.12
Effectiveness of zidovudine and related compounds is based on DNA chain termination due to the absence of the 3'-hydroxyl group.
In pyrophosphorolysis the viral reverse transcriptase enzyme complexed with the dideoxynucleoside monophosphate (ddNMP)-terminated primer binds pyrophosphate or ATP, results in removal of chain-terminating ddNMP, causing an unblocked DNA chain.
Binding pyrophosphate or ATP drives the reverse reaction. (http://www.ncbi.nlm.nih.gov/pubmed/12069972 )12
When used as a single antiretroviral drug, zidovudine is associated with clinical resistance has mutations gradually develop.1
About 30% of patients experience zidovudine resistance after 1 year of zidovudine monotherapy.
![]() Prolonged therapy, however, results in cross-resistance to
various nucleoside analogues.
Prolonged therapy, however, results in cross-resistance to
various nucleoside analogues.
A mutation at codon 69, a threonine to serine mutation, induces cross-resistance to "all available nucleoside and nucleotide analogues".1
Administration of lamivudine or emtricitabine results in a particular mutation at codon 184 (a methionine to valine substitution) in the viral reverse transcriptase gene that restores zidovudine sensitivity.10
![]() Combination
treatment of zidovudine and lamivudine has been shown to
extend long-term plasma HIV RNA suppression compared to zidovudine by
itself.13
Combination
treatment of zidovudine and lamivudine has been shown to
extend long-term plasma HIV RNA suppression compared to zidovudine by
itself.13
Consequently, zidovudine may be combined with lamivudine in the clinical setting.1
Pharmacokinetics:1
Following oral administration, zidovudine is rapidly absorbed, reaching peak plasma levels in about an hour.
The elimination t½ for the parent compound (prodrug) is about an hour, which is shorter than that of the intracellular triphosphate active compound by about a factor of four (i.e. about 3-4 hours).
Zidovudine exhibits first-pass hepatic metabolism (phase II glucuronidation), reducing bioavailability to about 65%.
The pharmacokinetic characteristics of zidovudine is not notably affected in pregnancy with the drug concentration in the newborn similar to that in the mother.
The parent agent, zidovudine, crosses the blood brain barrier fairly well with a CSF to plasma ratio (cerebrospinal fluid: plasma ratio) of about 0.6.
Zidovudine can be detected in breast milk, fetal tissue and semen.1
Adverse Effects:7
The most common zidovudine adverse effect is myelosuppression associated with macrocytic anemia (about 2%) or neutropenia (about 5%).7
Some adverse effects develop initially and then resolve during continued treatment.
Examples of these effects include headaches, insomnia, and gastrointestinal issues.
Lipoatrophy appears more frequently in patients receiving zidovudine or other thymidine analogue agents.
|
|
|
Less frequently observed toxicities include thrombocytopenia, nail hyperpigmentation and myopathy.
CNS effects may be noted at high doses, presenting as anxiety, confusion and tremor.
Co-administration of zidovudine with certain other agents result in increased zidovudine serum concentrations.
Elevated zidovudine in this setting can occur by:
Reduction of first-pass metabolism or by
Decreased drug clearance.
|
Probenecid |
Methadone |
Phenytoin |
|
|
Fluconazole |
Atovaquone |
Valproic acid |
Lamivudine |
By contrast, zidovudine administration may reduce phenytoin levels.
Increased hematological toxicity may be observed upon co-administration of other myelosuppressive agents such as:
Ganciclovir
Ribavirin
Cytotoxic drugs
As discussed earlier, combination protocols containing zidovudine and stavudine should be avoided because of drug-drug antagonism (competition for intracellular phosphorylation).7
![]() Therapeutics:1
Therapeutics:1
![]() Zidovudine (Retrovir,
AZT) is approved (FDA) both for treating adults and children infected
with HIV and for preventing mother-to-child HIV transmission.1
Zidovudine (Retrovir,
AZT) is approved (FDA) both for treating adults and children infected
with HIV and for preventing mother-to-child HIV transmission.1
Given the long time and broad experience with zidovudine, zidovudine remains recommended for post-exposure prophylaxis in HIV-exposed health-care workers.
This conclusion has been described in a 2005 Centers for Disease Control and Prevention (CDC) report (here) which has been slightly modified in 2013 (here).
Zidovudine is available as oral tablets, capsules, syrup and solution, including a solution suitable for intravenous injection.1
|
|
|
|
|
|
Zidovudine is also available in coformulation tablets with other antiretroviral drugs.1
![]() For example,
zidovudine may be compounded with lamivudine (Combivir) or with
lamivudine and abacavir (Trizivir).
For example,
zidovudine may be compounded with lamivudine (Combivir) or with
lamivudine and abacavir (Trizivir).
As monotherapy, zidovudine decreases perinatal HIV transmission risk by about 67%.
In this application use of zidovudine with other antiretroviral drugs is even more effective in preventing HIV perinatal transmission.1
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |