|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiretroviral Drugs Used in Treating HIV Infection
→Non-nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NNRTI): (continued)
|
Etravirine (Intelence) |
|
|
|
|
Etravirine (Intelence) inhibits HIV-1 replication at low concentration, in the nanomolar range.
The concentration of etravirine required to inhibit HIV-1 reverse transcriptase by 50% (IC50) is approximately 2.5 nanomolar (nM).
As with other members of the NNRTI antiretroviral drug class, etravirine does not show inhibitory activity against HIV-2.
![]() An
important and unique aspect of etravirine pharmacology is that the drug
inhibits reverse transcriptase otherwise resistant to other NNRTI drugs.
An
important and unique aspect of etravirine pharmacology is that the drug
inhibits reverse transcriptase otherwise resistant to other NNRTI drugs.
For example, etravirine is not affected by the K103N mutation or by K103N/Y181C mutation pair, which mediates resistance to efavirenz, nevirapine and delaviridine.
Multiple mutations appear required to confer etravirine drug resistance with virologic failure.
Mutations and Resistance to Etravirine (Intelence)
Although a number of mutations have been identified in HIV-1 reverse transcriptase that can limit or eliminate sensitivity to etravirine's antiretroviral efficacy, several mutations appear particularly effective in conferring resistance.8
As described below, these mutations occur at amino acid 100, 101, and 181.8
There are numerous other sites where mutations have been identified, including amino acid at 90, 98, 106, 138, 179, 190 and 230.
At site 100 the wild type amino acid leucine is substituted by an isoleucine.
At amino acid 101, a lysine is replaced by glutamate, histidine, or proline.
At amino acid 181, the wild type amino acid tyrosine is replaced by either cysteine, isoleucine, or valine.8
Other studies suggest that the glycine to alanine mutation at site 190 is also an important resistant muation.6
A concern and possible limitation of etravirine resistance investigations is that many studies consider etravirine resistance in patients also receiving darunavir, a possible confounding factor.1
|
|
|
|
|
|
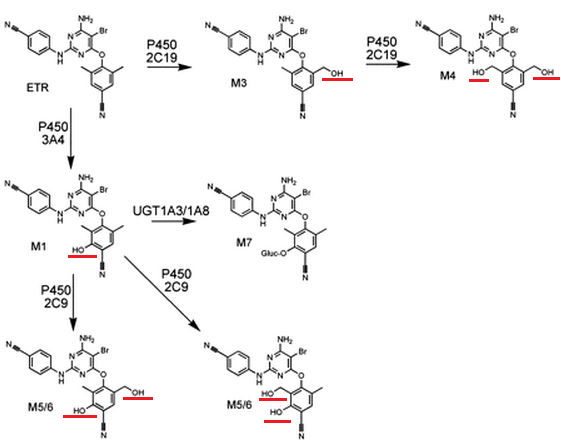
Etravirine (Intelence) is administered orally and is then rapidly absorbed.1,7
Highest plasma concentration occurs about three hours following administration.
Administration of etravirine with food results in increased Area Under the Curve (AUC) levels and as a result of etravirine should be administered with a meal.
Etravirine is eliminated from the body utilizing the cytochrome P450 microsomal metabolizing system.1,7
As noted earlier for other agents, certain enzyme isoforms of cytochrome P450 are more important than others in metabolizing certain drugs.
In this case, three cytochrome P450 isoforms appear most important: CYP3A4, CYP2C9, and CYP2C19.
Metabolism by these agents results in methyl-and dimethyl-hydroxylated metabolites.1
 |
|
![]() A
variety of pharmacokinetic-related drug interactions occur because
etravirine not only induces CYP3A4 but also inhibits CYP2C9 and CYP2C19.1,7
A
variety of pharmacokinetic-related drug interactions occur because
etravirine not only induces CYP3A4 but also inhibits CYP2C9 and CYP2C19.1,7
![]() For
example, within the antiretroviral drug category, etravirine
decreases efavirenz and dolutegravir serum levels while increasing
fosamprenavir serum levels.
For
example, within the antiretroviral drug category, etravirine
decreases efavirenz and dolutegravir serum levels while increasing
fosamprenavir serum levels.
Another example involves antifungal drugs in which, etravirine administration on one hand, reduces itraconozole and ketoconazole levels and on the other increasees voriconazole levels.
Unmetabolized etravirine is not found in the urine.
Elimination t1/2 for etravirine is about 40 hours, allowing for twice-daily dosing.
This dose frequency is an example of how newer drugs have dramatically reduced the extent of "pill burdens" when compared with older formulations.1
The most frequently observed adverse effects associated with etravirine administration include rash, diarrhea and nausea.7
Rash is typically self-resolving within about two weeks.
The rash is rarely severe.7
In at least one clinical trial involving an etravirine + darunavir combination protocol, rash was the only side effect that appeared more common with etravirine when compared with placebo.1
In that instance rash was observed within several weeks following treatment initiation and lasted about two weeks.
About 2% of patients in this study discontinued etravirine as a consequence of rash.1
![]() However,
more serious rash such as Stevens-Johnson syndrome and toxic
epidermal necrolysis has been described.1
However,
more serious rash such as Stevens-Johnson syndrome and toxic
epidermal necrolysis has been described.1
Etravirine was not associated with either neuropsychiatric or hepatic adverse effects, relative to placebo administration.1
However, laboratory anomalies following etravirine administration may include increases in serum cholesterol, triglyceride, anhepatic transaminase levels.
Glucose levels may also be elevated.7
Increases in transaminase levels appear more likely in patients with either hepatitis B or hepatitis C co-infection.7
As noted earlier, since etravirine induces CYP3A4 as well as glucuronosyltransferases and inhibits CYP2C9 and CYP2C19 pharmacokinetic-drug interactions are expectable.1
Some combinations do not require etravirine dose adjustments.1
Examples of these combinations include darunavir + ritonavir, lopinavir + ritonavir, and saquinavir + ritonavir.
![]() By contrast the dose of maraviroc likely requires adjustment if used in
combination with etravirine.
By contrast the dose of maraviroc likely requires adjustment if used in
combination with etravirine.
Furthermore, etravirine, pending better information concerning possible dose adjustment requirements, probably should not be administered with tipranavir + ritonavir fosamprenavir + ritonavir or atazanavir + ritonavir.
![]() Finally,
etravirine should not be combined with efavirenz, delavirdine, or
nevirapine.
Finally,
etravirine should not be combined with efavirenz, delavirdine, or
nevirapine.
At present using two NNRTI-class drugs in combination is not recommended for any treatment protocol.9
![]() Etravirine (Intelence) is not in the recommended category for
antiretroviral-na´ve patients as a result of insufficient clinical data.9
Etravirine (Intelence) is not in the recommended category for
antiretroviral-na´ve patients as a result of insufficient clinical data.9
As a consequence this agent is approved for administration only in "treatment-experienced" HIV-infected adult patients.1
In treatment-experienced patients with baseline therapy of a darunavir + ritonavir combination, adding etravirine increase the percentage of patients achieving a plasma HIV-RNA reference level of <50 copies/cc as determined at week 48 of treatment.
Patients receiving etravirine along with the other agents exhibited improved mean CD4+ T cell counts, also determined at week 48.
Week 48 virologic responsiveness appear dependent on the extent of baseline etravirine-resistance mutations.1
![]() With respect to initial combination protocols in the antiretroviral-na´ve
patient, five regimens have been described.9
With respect to initial combination protocols in the antiretroviral-na´ve
patient, five regimens have been described.9
Four of the five are based on integrase strand transfer inhibitors and one protocol is based on a ritonavir-boosted protease inhibitor.9
The details of these recommended protocols will be described in the section on integrase strand transfer inhibitor drugs.
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |