|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiretroviral Drugs Used in Treating HIV Infection
→Non-nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NNRTI): (continued)
|
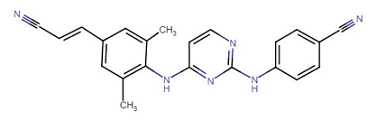
Rilpivirine (Endurant) |
 |
|
|
Rilpivirine (Endurant) is classified as a second generation NNRTI (non-nucleoside reverse transcriptase inhibitor) that has proven useful (in combination with other drugs) in HIV antiretroviral drug therapy for treatment-naïve adult individuals.10
Rilpivirine has been combined with other agents such as tenofovir disoproxil fumarate and emtricitabine for management of HIV infection.9
Rilpivirine in combination with tenofovir disoproxil fumarate and emtricitabine was until recently described as a "Recommended regimen."9
However in the April, 2015 revision this combination was reclassified to the "Alternative or Other category."
The protocol, applies to patients with baseline HIV RNA <100,000 copies/cc or with CD4+ T lymphocytes (CD4) counts of >200 cells/mm3.9
Mutations and Resistance to Rilprivirine (Endurant):
|
|
|
|
|
Probably the most common rilpivirine NNRTI resistance mutation is located at codon 138 reflecting an E138K transition.8,9
This transition corresponds to a replacement of the wild-type, naturally occurring glutamate amino acid for a lysine.
As noted, other mutations have also been documented.
The E138K mutation results in a generalized NNRTIs resistance which includes a resistance to etravirine.8,9
Rilpivirine treatment may also select for K101E, Y181C and V189I resistance mutations.
The K101E describes a transition from the naturally occurring, wild-type lysine to a glutamate amino acid.
Y181C represents a transition from the naturally occurring, wild-type tyrosine to a cysteine amino acid.8,9
Rilpivirine administration should be with a high-fat meal (>400 kcal).6.7
Oral absorption of rilpivirine can be notably reduced if the patient is also taking acid-reducing drugs, including and acids and H2-receptor blockers.
Accordingly, rilpivirine administration along with proton-pump inhibitor drugs is a contraindication.
Dosage adjustment is not recommended for patients with renal dysfunction even if the patient is requiring hemodialysis or peritoneal dialysis.
Furthermore in those patients with only mild-to-moderate liver insufficiency, no dosage adjustment is apparently required.
In those individuals with severe liver dysfunction, the issue of dosage adjustment remains to be resolved.7
The cytochrome P450 microsomal metabolizing system is responsible for rilpivirine metabolism.6.7
The primary P450 isoform involved is CYP3A4.
Rilprivine plasma half-life is estimated to be approximately 2 days (t½≈ 50 h).
Rilpivirine clearance is influenced by drugs which increase or decrease the concentration of CYP3A4.
For example, efavirenz and rifamycins reduce rilpivirine plasma concentrations.
By contrast, administration of protease inhibitors and azole antifungals leads to higher rilpivirine plasma levels.
![]() Examples of drugs which are contraindicated for use concurrently
with rilpivirine include:7
Examples of drugs which are contraindicated for use concurrently
with rilpivirine include:7
Carbamazepine
Phenobarbital'
Phenytoin
Proton pump inhibitors (as noted above)
Dexamethasone,
Rifabutin
Rifampin
Rifapentine
St. John's wort.
Examples of adverse effects associated with rilpivirine administration include:6
Depression
Headache
Insomnia
Rash
Rilprivirine-induced rash appears notably less common compared to efavirenz.
At high, "supratherapeutic" doses prolongation of cardiac QTc may occur.6
Based on two international phase 3 randomized, placebo controlled clinical trials (ECHO and THRIVE), the increase in the QT interval was about 11 ms from baseline.12
Few adverse effects possibly related to conduction anomalies or to rate and rhythm disturbance were noted.
Based on in vitro studies, the most likely mechanism accounting for rilpivirine-induced changes in the QT interval was related to potassium channel blockade.12
Rilprivirine is classified as one agent in a multiagent protocol in the alternative or other category.9
Rilprivirine may be used in treatment-naïve patients with with baseline HIV RNA <100,000 copies/cc or with CD4+ T lymphocytes (CD4) counts of >200 cells/mm3.9
Rilpivirine is thus used in combination with at least two other antiretroviral agents.7
A fixed-dose formulation including emtricitabine, tenofovir and rilpivirine has been marketed.7
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |