Medical Pharmacology Chapter 43: Adult Cardiac Procedures
|
|
Medical Pharmacology Chapter 43: Adult Cardiac Procedures
|
|
|
|
|
|
Cardiac Conduction System: Review
Structure: SA node
Disk shaped -- dimensions = 15 x 15 x 2 mm
Location -- in the sulcus terminalis (junction of the superior vena cava and right atrium; posterior cardiac aspect)
Anatomical origin -- right-sided embryological structures {note consequence -- primarily right-vagal nerve innervation dominance}
Blood supply: usually (60%) from the right coronary
Intrinsic pacemaker beats per minute (bpm)-- idioventricular pacemaker rates may be insufficient to ensure adequate cardiac output (note below)
SA node: 60-100 bpm
Atrial cells: 55-60 bpm
AV node: 45-50 bpm
HIS bundle: 40-45 bpm
Bundle branch: 40-45 bpm
Purkinje cells: 35-40 bpm
Myocardial cells: 30-35 bpm
SA nodal action potential characteristics:
"Slow-response" type, consistent with limited fast-sodium channel activation involvement
Characteristic phase 4 depolarization (unstable membrane potential drifting towards threshold-- phase 4 depolarization slope influenced by sympathetic/parasympathetic stimulation as well as other factors.
|
|
|
SA & AV nodal, slow response, fibers exhibit Ca2+-mediated inward currents which are responsible for depolarization
The channel type appears to be of the L-type Ca2+channel, voltage-gated, typically activated membrane potentials > -40 mV
L-type calcium channel inactivation is dependent on (a) cell membrane potential, [ Ca2+], and time
L-type calcium channel antagonists include:
Benzothiapines (e.g. diltiazem (Cardiazem))
Dihydropyridines (e.g. nifedipine (Procardia, Adalat) & nitrendipine
Phenylalkylamines (e.g. verapamil (Isoptin, Calan)
SA nodal overdrive suppression
Pacemaker cellular automaticity is suppressed following a period during which action potentials are driven (typically by external pacing) at a rate higher than the normal intrinsic pacemaker frequency
Suppression of ectopic pacemakers may occur as a result of overdrive suppression, allowing the normal SA nodal pacemaker to resume control of heart rate.
In "sick-sinus syndrome" rapid heart rates inhibit normal SA nodal pacemaker activity and consequently can lead to periods of asystole.
Mechanism: Related to the activity of Na/K ATPase
At higher heart rates enhanced sodium pump activity results in cell membrane hyperpolarization because 3 sodium ions are extruded for 2 potassium ions entering the cell
As a consequence of cellular hyperpolarization, diastolic depolarization (automaticity) will take longer to reach threshold
When overdrive ends, the Na/K ATPase pump activity does not immediately returned to normal does favoring the hyperpolarized membrane potential, which is a consequence suppresses normal nodal automaticity for a brief time.
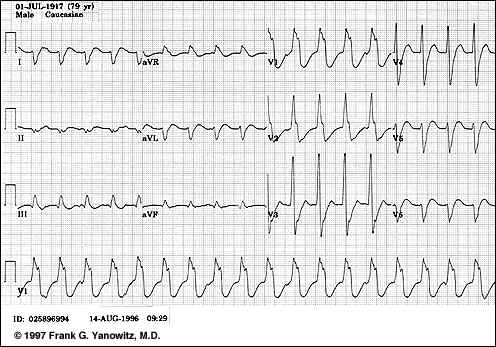
The relationship between cardiac output and heart rate provides the basis for rapid intervention for management of either tachycardia or bradycardia
Bradycardia: In the case or bradycardia, secondary to sick sinus syndrome or due to a very slow idioventricular rate {associated with complete atrioventricular block}, ventricular filling may be limited despite prolonged diastole, perhaps due to pericardial noncompliance. This reduction in diastolic filling may be sufficient to prevent compensation by increased stroke volume.
Tachycardia: With supraventricular or ventricular tachycardias, cardiac output may be dangerously low as a result of inadequate filling time
In tachycardic patients, the filling time may be sufficiently restricted such that a slight further reduction in filling time may have a disproportionately serious reduction in filling volume
Drug intervention to reduce heart rate may be effective; however in an emergency setting DC cardioversion may be more appropriate.
 |
|
Internodal pathway
Three internodal tracts provide input to the AV node {i.e., primary function is transmission of SA nodal pacemaker impulses to the AV node}
Internodal pathways are localized in the inter-atrial septum and right atrial walls
Three functional AV nodal zones:
AN zone: joining atrium to the node
N (nodal) region
NH zone: joining the node to the bundle of His
Significance: functional AV nodal zones:
Delay between atrial impulse transmission to distal conduction components-- due to "N" regions & "AN" regions
This delay allows for adequate ventricular filling subsequent to atrial contraction {associated with the PR ECG interval}
Structure: AV node
Button-shaped structure (22 x 10 x 3 mm)
Location:
Right posterior side of interatrial septum near coronary sinus ostium (coronary sinus = largest cardiac vein), near the tricuspid valve septal leaflet
Embryological development -- left-sided, accounting for left-sided domination of AV nodal vagal innervation
Function: reduces atrial-ventricular conduction, thus allowing time for ventricular optimal filling
AV node blood supply provided by the right coronary artery
Bundle of His:
AV nodal fibers proceed to the bundle of His
Bundle of His extends subendocardially along the right side of the interventricular septum, then divides, continuing as the right bundle branch
Singular communication pathway between atria and ventricles
The left bundle branch also arises from the bundle of His, also passing through the interventricular septum and further dividing into a thin anterior fascicle as well as a thick posterior fascicle proceedings subendocardially along the left side of the interventricular septum. (see diagram below)
|
|
|
Left bundle branch (LBB):
Initiation point: end of the His bundle
Progresses through the interventricular septum
LBB fibers innervate:
the left ventricle
the interventricular septum
Cardiac regions innervated by the LBB are the first to depolarize
Ending point: at the beginning of the left anterior and left posterior fascicles (LAF & LPF)
The Purkinje fiber network, deriving from the right bundle and the two left fascicles, allows impulse conduction throughout the ventricle
Rapid impulse conduction associated with Purkinje fibers (secondary to thicker cellular diameter) permits rapid action potential propagation through the myocardium, thus coordinating contraction
Contraction sequence, as a result of action potential propagation through Purkinje fibers
Interventricular myocardial tissue (septum) & papillary muscle
Septal contraction provides an "anchor" for the heart as the ventricular free wall begins contracting and papillary muscle tightening prevents tricuspid & mitral valve prolapse during early systole
Muscle fiber contraction proceeds outward from the endocardial region to the epicardial surface
The right ventricle contracts before the left ventricle secondary to differences in wall thickness (differences in output from the left vs. right-side are compensated in accord with Starling's Law)
Overview
Automaticity, a SA nodal normal characteristic and a characteristic of specialized His-Purkinje system fibers as well as some specialized atrial fibers is defined as a cardiac cellular property which causes spontaneous cell membrane depolarization.
This depolarization occurs during phase 4 of the action potential.
The primary characteristic of automaticity is that the resting membrane potential is not stable but rather decreases (towards 0 millivolts (mv)) until threshold is reached an action potential generated
In those normal cells which would be expected to exhibit automaticity, specific ionic currents have been identified as responsible. These currents are:
an inward current (If), probably carried by Na+ which tends to depolarize the membrane
a second inward current carried by Ca2+, (ICa2+), which is also depolarizing
and a third outward current carried by K+ (IK+), the conductance of which tends to decrease during phase 4, those leading to a net depolarizing effect. This outward current tends to oppose the inward (If) and (ICa2+) currents' depolarizing influence. Thus the time-dependent decrease in IK+ is an important factor leading to the positive slope associated with phase 4 depolarization.
|
|
Physiological Factors Affecting Automaticity:
Autonomic effects: Adrenergic
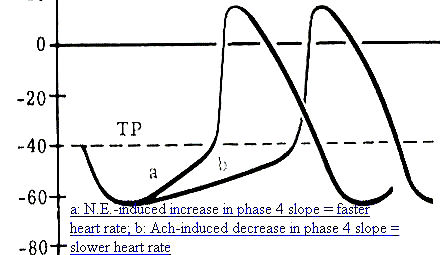
Adrenergic neurotransmitters (e.g. norepinephrine) increase (If) and (ICa2+) and IK+) currents with predominant effects on (If) and (ICa2+). Predominant effects on the depolarizing (If) and (ICa2+) explains why adrenergic stimulation increases heart rate [increasing phase 4 slope means that action potential threshold is reached more frequently (per minute), thus resulting in a faster rate]
Autonomic effects : Cholinergic
Cholinergic effects, acetylcholine-mediated, result in an increase in K+conductance (gK+), an effect which is hyperpolarizing (the membrane potential moves further away from threshold). Membrane hyperpolarization results in an increased length of time required for threshold to be reached during phase 4 depolarization -- a consequence of this effect is that the heart rate will be reduced.
In addition, acetylcholine depresses the depolarizing, (If) and (ICa2+) currents-- also contributing in this way to a reduced phase 4 slope, consistent with the reduced heart rate following increased vagal activity.
|
|
|
 |
|
Factors that increase automaticity:
β-adrenergic receptor activation
* 1 Hypokalemia, reduced intracellular potassium decreases outward K+ movement which normally counteracts phase 4 depolarization (see above).
Recall that in phase 4 depolarization inward depolarizing currents are partially offset by outward potassium ion movement; therefore, in the hypokalemic state the depolarizing currents will be more dominant and as a result increased the slope of phase 4.
With an increased phase 4 slope, the threshold potential is reached more easily
This mechanism is also partially responsible for the pro-arrhythmic activity associated with toxic digoxin (Lanoxin, Lanoxicaps)/digitoxin (Crystodigin) levels
Recall that in digitalis toxicity, probably due to inhibition Na/K ATPase, there is an accumulation of intracellular Na+and Ca2+.
Consequently, intracellular accumulation of positively charged ions tends to decrease the membrane potential (less negative), bringing it closer to threshold.
|
|
|
|
|
|
2 Stretch-induced depolarizations are known to cause arrhythmias.
Furthermore stretch-activated ion channel have been identified and their electrophysiological properties characterized.
Mechanosensitive ion channels were first discovered in skeletal muscle with subsequent identification in cardiac cells, bone, and in most other types of cells including those in bacteria and plants
See Sachs F: 1992. Stretch sensitive Ion channels: an update. In Corey DP, Roper SD, eds. Sensory Transduction, New York, Rockefeller University press, Soc Gen Physiol. pp 241-260 and GCL Bett and F. Sachs (1997) Cardiac Mechanosensitivity and Stretch-Activated Ion Channels, Trends in Cardiovascular Medicine 7: 4-8.
3 Injury Currents: Consider the 12-lead ECG: During an antianginal episode, ST segment depression may be observed. Normally the ST segment is associated with phase 2 of the myocardial action potential.
Phase 2 is typically isoelectric (no significant membrane potential change is occurring-see below)
|
|
With ischemia, the resting membrane potential in the ischemic region is reduced compared to surrounding healthy tissue. As a consequence of this membrane potential difference, current flows between the two regions. This current is referred to as an injury current and is reflected by deviations in the ECG ST segment
For subendocardial ischemia, the ST vector is towards the ventricular cavity, resulting in surface leads reporting ST depression; however, in transmural ischemia, the ST vector is oriented towards the surface lead, causing ST segmental elevation {Biomedical Sciences 245B, Virginia Commonwealth University: Pathophysiology of Disease: Ischemic Hard Disease (Krishnan)
4 Acidosis: Tends to suppress normal automaticity
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |