|
|
|
Structure-Activity Relationships continued
1,2aMorphine, as noted earlier is a rigid five-ring configuration.
The phenylpiperidine component forms the crossbar of the "T", whereas the hydroxylated aromatic ring provides the vertical component.
Reducing the number of fused rings corresponds to the transition of phenanthrenes to morphinans (four-rings) then on to the benzomorphans (three rings) then the phenylpiperidines (two rings) and lastly the tyramine moiety of endogenous opioid peptides characterized by a single hydroxylated ring. all of the use classes possess morphine-like pharmacological activity and opioid receptor models have been suggested based on these structures.
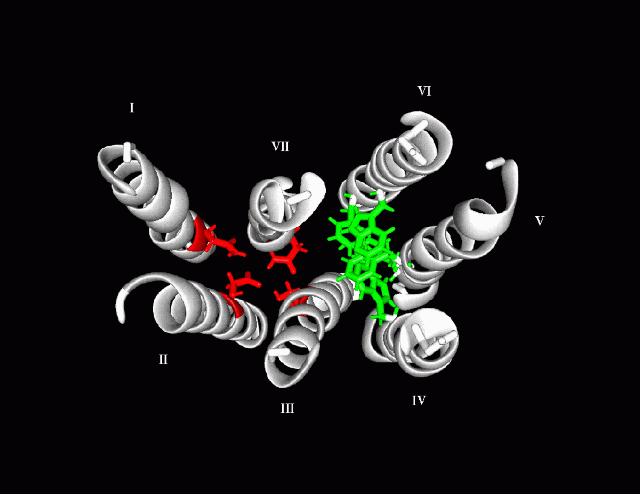
11Models exhibit 2 aromatic binding sites with 1 anionic (negative) site which binds to the positively charged nitrogen.
|
|
|
![]()
|
![]()
|
|
![]()
|
Analgesia Mechanisms
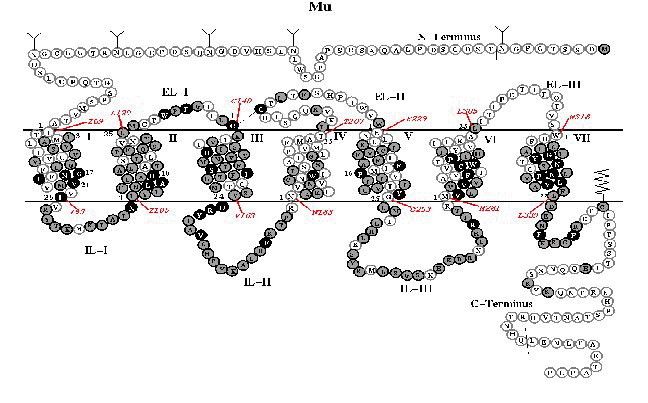
1, 1h,1iAnalysis of opioid receptor localization, genetic expression, and biophysical functional characteristics has been central in the evolution of fundamental understanding of opioid analgesic mechanisms.
Identification of certain sites is particularly important in understanding opioid action.
These sites have included the amygdala, mesencephalic reticular formation, periaqueductal gray and rostral ventral medulla.
At the level the spinal cord, the substantia gelatinosa is an important opioid site.In addition to receptor localization, designation of a particular anatomical location is important depends on other experimental results as well.
The question is not whether an opioid may bind at a particular anatomical site but whether such binding has a physiological consequences referencing the particular endpoint of analgesia.
Periaqueductal gray area is a region in the brainstem at which morphine injection, or electrical stimulation has been demonstrated to cause analgesia.1j
Furthermore, analgesia so induced is blocked by naloxone, the classical opioid antagonist.
Opioid activity at periaqueductal gray regions induces effects down the anatomical line.
Direct connections to the rostral ventromedial is one mechanism by which opioid effects may be propagated.
Furthermore, this medullary region can affect nociceptive transmission neurons located in the spinal cord dorsal horn.
Full analgesic effects of opioids require anatomical connections between the brain and spinal cord.
Localized opioid administration at the level the cord produces expectable analgesic effects; however, effects are magnified by activation at remote CNS opioid receptor rich sites.1k
Spinal mechanisms: opioid activity also involves direct, localized spinal action in addition to the above describe descending pathways effects.
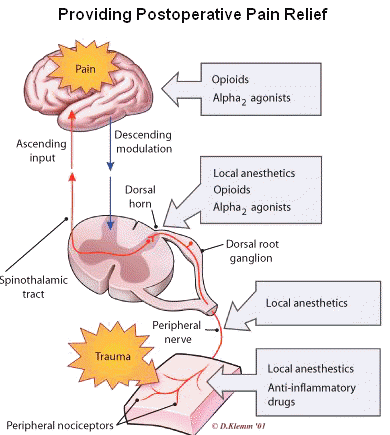
At the synaptic level, opioids have presynaptic (modulatory) as well as postsynaptic activity. Some of these sites are represented in the figure below:
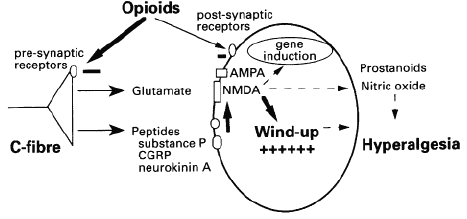 |
4As suggested in the figure above, a number of substances in addition to the opioids have an important role in affecting spinal cord pain transmission.
In addition to excitatory amino acids such as glutamate and aspartate, peptides are important in nociceptive transmission.
The category of peptides viewed as especially important is the tachykinin group.
In addition, other important peptides include calcitonin gene-related peptide (CGRP), somatostatin, vasoactive intestinal peptide, galanin, bombesin and neurotensin.
Neurokinins:
Substance P is the most prominent member of this group, having been associated with nociception for some time.
Substance P associated with the dorsal horn originates from primary afferent fibers and intrinsic neurons along with contributions from descending fibers.
Three subclasses of neurokinin or tachykinin receptors have been identified:
neurokinin-1
neurokinin-2 and
neurokinin-3.
Substance P appears to be the favored tachykinin at neurokinin-1 receptor sites.
Neurokinin receptors appear to be postsynaptic to the afferent fiber terminals located in laminae I, II, and X of the spinal cord dorsal horn.
Substance P release into the dorsal horn by noxious stimuli has been demonstrated.
Peripheral inflammation is characterized by an increase in substance P release as well as an increase in the release of a related peptides, neurokinin A.
Neurokinin A release is sufficiently pervasive such that most of the dorsal horn can be influenced.
|
Calcitonin gene related peptide (CGRP): This afferent peptide, released by noxious stimulation, is excitatory to dorsal horn neurons.
13CGRP (37 amino acid peptide)
Somatostatin:
The substance is present in small diameter cells associated with dorsal root ganglia and in afferent terminals in the spinal cord substantia gelatinosa.
Painful thermal stimulation increases somatostatin release within the dorsal horn.
Somatostatin-application has a direct electrophysiological effect on dorsal horn neurons, causing hyperpolarization with a reduction in firing rate (spontaneous).
This hyperpolarization effect suggests that somatostatin has an inhibitory role in the dorsal horn, leading to the suggestion that this peptide may be an important afferent inhibitory transmitter.
At somewhat higher doses than required for this hyperpolarization effect, pathological manifestations have been recorded, including motor dysfunction and paralysis.
Excitatory amino acids:
As described in more detail later, glutamate has an important presence in the spinal cord deriving permits concentration in both myelinated and unmyelinated primary afferent fibers as well as its presence in intrinsic interneurons and projection neurons.
Excitatory amino acid biological effects are mediated through the NMDA receptor (described below in terms of different forms).
Peripheral sensory fibers often contain both glutamate and aspartate; furthermore, those fibers containing substance P also often contain glutamate.4a
This finding was considered important because it suggests that glutamate release from these intrinsic neurons pursuant to a noxious stimulant would also be associated with peptide release.
![]()
High densities of opioid receptors have been identified in the substantia gelatinosa.
Furthermore, application of opioids to these receptor fields induces significant analgesic responses.
Primary sensory
neuronal release of substance P is inhibited by opioid agonists (![]() ,
,![]() -,
and
-,
and ![]() -
agonist types).
-
agonist types).
Substance P release inhibition is a probable mechanism for opioid analgesia.
Some prominent sites of opioid action are summarized in the diagrams below:
 |
 |
1Other localization sites for opioid receptors include: nucleus receiving pain fibers from the face and hands by way of the 5th, 7th, 9th and 10th branches of the cranial nerves.
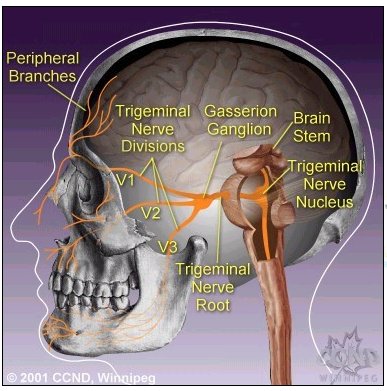
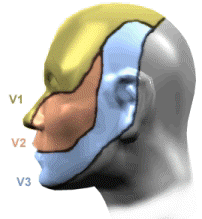
Fifth Cranial Nerve: Trigeminal Nerve
8"The three divisions of the trigeminal nerve come together in an area called the Gasserion ganglion.
From there, the trigeminal nerve root continues back towards the side of the brain stem, and inserts into the pons.
Within the brain stem, the signals traveling through the trigeminal nerve reach specialized clusters of neurons called the trigeminal nerve nucleus.
Information brought to the brain stem by the trigeminal nerve is then processed before being sent up to the brain and cerebral cortex, where a conscious perception of facial sensation is generated"
 |
 |
7Seventh Cranial Nerve: Facial
 |
 |
Ninth Cranial Nerve: Glossopharyngeal
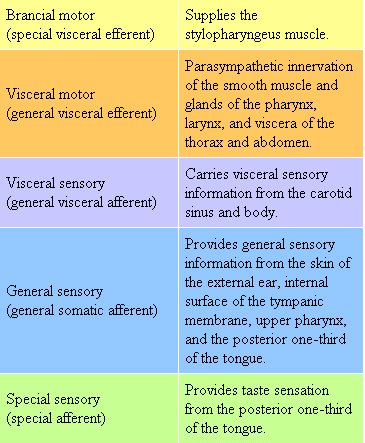 |
- Tenth Cranial Nerve: Vagus
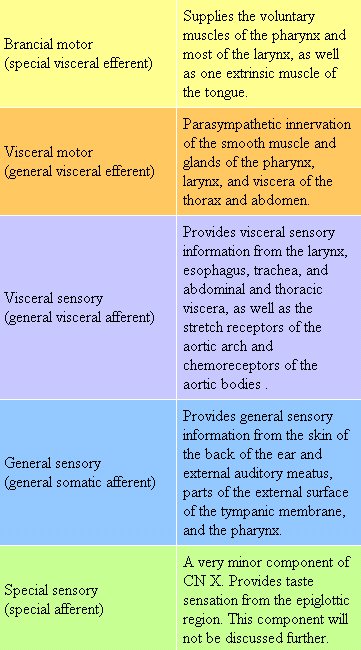 |
1Dorsal horn neuronal excitation is inhibited by opioids -- excitation due to painful, sharp stimuli.
Sensations
mediated by type A-![]() fibers are diminished. EPSP summation (excitatory postsynaptic
potential summation) is attenuated by opioids at the level the dorsal
horn.
fibers are diminished. EPSP summation (excitatory postsynaptic
potential summation) is attenuated by opioids at the level the dorsal
horn.
Furthermore, consequence of this effect is the blockade of dull persistent pain (C-fiber transmission).
![]() The summation effect is
apparently easier to block them treat them as a rationale behind the idea of
preemptive analgesic as well as a
possible explanation with respect to why patient postsurgical responses are
more readily managed with opioids before rather than after
stimulation.
The summation effect is
apparently easier to block them treat them as a rationale behind the idea of
preemptive analgesic as well as a
possible explanation with respect to why patient postsurgical responses are
more readily managed with opioids before rather than after
stimulation.
Opioids reduce excitatory threshold reduction and receptive field expansion at the spinal cord level by means of affecting second-order neurons.
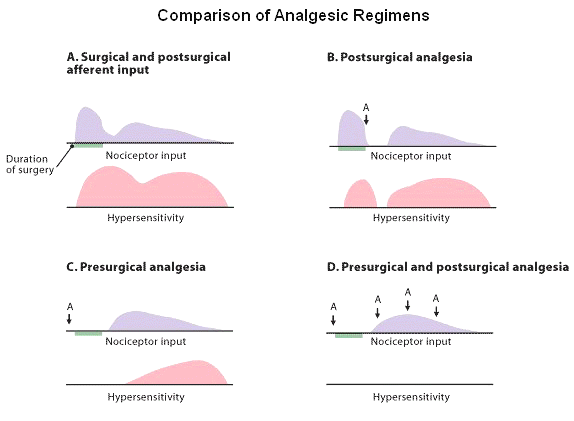 |
|
1Tolerance: Reduced responses to opioids occur over time.
The molecular basis for tolerance may be in uncoupling of the drug-receptor linkage.
A reduced number receptors and/or a reduction in agonist affinity or disruption between the activated receptor and intracellular second messenger systems may account for reduced opioid effects.
Other cellular processes may adapt to the presence of opioids by altering gene expression in a manner that restores cyclic AMP to normal levels [opioid-mediated effects on intracellular second messenger systems may have the initial effect of increasing adenyl cyclase activity leading to increased synthesis of cyclic AMP].
Changes in the opioid responses can be secondary to alteration of messenger RNA (mRNA) expression levels and be also modulated by receptor phosphorylation states (a common way of altering protein function is by controlling the extent of phosphorylation).
Another suggestion has been an opioid tolerance
occurs as a result of the switch from mainly opioid receptor
inhibitory Gi![]() to stimulatory G
to stimulatory G![]()
![]() signaling.9 Recall here that opioid receptors are
mainly couple to adenyl cyclase by means of the Gi
signaling.9 Recall here that opioid receptors are
mainly couple to adenyl cyclase by means of the Gi![]() subunit of G proteins
subunit of G proteins
Cross-tolerance which is seen so commonly within the sedative-hypnotic drug categories, is not significantly observed between agents that activate opioid receptor subtypes.
The most potent opioid agonists e.g. sufentanil, which may be associated with large receptor reserves are least likely to exhibit tolerance.
![]()
Systems Physiology in Nociception and Antinociception
Synaptic Activity of Transmitter/Modulatory Systems in Nociception and Antinociception
Endogenous Opioids
![]()
10Lateral and Medial Pain Transmission Systems (illustration by Seward Hung)
|
|
|
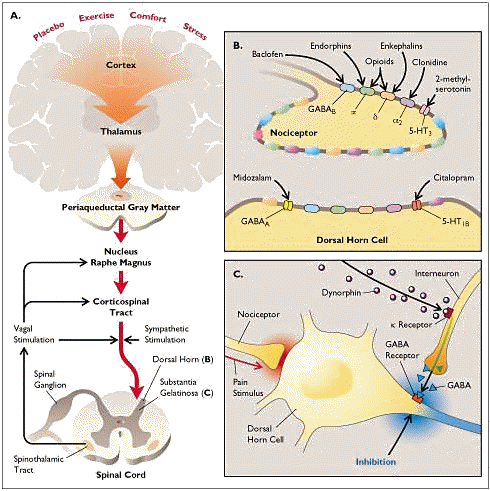 |
10Responses to pain induce activity in antinociceptive pathways. (note the figure above)
This activity begins when pain information transmitted by the spinothalamic tract reaches the brainstem and thalamus (A above).
Activation of periaqueductal gray and the nucleus raphe magnus induces endorphin and enkephalin release and binding to "opioid" receptor systems.
Sympathetic and parasympathetic influences within the spinal cord facilitate inactivation of antinociceptive pathways.
Most of the endorphin and enkephalin receptors (70%) are localized presynaptically, substantial pain signal attenuation occurs before information reaches the dorsal horn (B above).
Such information may be further attenuated by enkephalin-induced dynorphin activity at the level the cord (C above).
Dynorphin activates
-type opioid receptors localized on inhibitory interneurons, activation of which induces release of the inhibitory neurotransmitter GABA.
The mechanism by which
-opioid receptor activation limits spinal cord cellular activity may be by means of closure of N-type Ca2+ channels.
Interaction of GABA with its receptor results in dorsal horn neuronal hyperpolarization thus impeding transmission of the pain information.
Reduction of visceral pain may occur particularly by this approach.
Enkephalin binds to
-type opioid receptors which appear on nociceptive neurons when they actively transmit pain information.
Furthermore, these receptors are often localized on presynaptic vesicles that contain neurotransmitter and following release receptor protein is incorporated into presynaptic membrane.
Active nociceptors, because of preferential binding, are therefore more sensitive than inactive nociceptive receptors to endogenous opiates.
This idea may be relevant in explaining how opioid analgesics appear to relieve ongoing pain but do not prevent sensing of pain subsequent to new injuries.
![]()