5Case study and analysis: chronic obstructive pulmonary disease
|
Wheezing and dyspnea raise the possibility of several disease processes including, bronchial asthma, acute left ventricular failure, tumor or angioedema causing upper airway obstruction, endobronchial disease including foreign body aspiration, neoplasms, bronchial stenosis, carcinoid tumors, recurrent pulmonary emboli, chronic bronchitis, eosinophilic pneumonias, chemical pneumonias, and on more rare occasions polyarteritis.
To distinguish asthma from these other possibilities generally is not challenging even though some of these diseases exhibit wheezing and dyspnea.
The patient's history indicating periodic attacks, establishes the episodic nature of the disorder which in combination with coughing, wheezing, and dyspnea favors the asthma diagnosis.
Allergic disease associated with the individual and family is also helpful in establishing the diagnosis.
In an individual who has had asthma for prolonged period, chronic obstructive lung disease may have developed and the patient may exhibit orthopnea (note the requirement of two pillows for this patient) and exertional dyspnea (again note the patient history). Left ventricular failure, reflecting basic cardiac pathology, exhibits pulmonary clinical manifestations such as pulmonary edema.
Differential diagnosis of asthma vs. left ventricular failure include the presence of moist basilar rales, blood-tinged sputum, peripheral edema, gallop cardiac rhythms and a probable history of cardiac disorders.
5Distinguishing between obstructive and restricted lung disease using spirometric methods
Restricted: in restricted disease such as pulmonary fibrosis or ankylosing spondylitis the forced vital capacity (FVC) is reduced secondary to reduced lung or chest wall expansion capabilities. Since airway resistance will be approximately normal, FEV1 will not be reduced in a manner proportional to the reduced FVC values. The FEV1/FVC ratio tends to be normal or somewhat elevated
By contrast, in obstructive lung disease, for example emphysema, the FEV1/FVC is significantly reduced due to high airway resistance. Furthermore, maximum breathing capacity (MBC) and maximum midexpiratory flow rate (MMEFR) will be reduced early in small airway obstructive disease. MMEFR corresponds to our earlier designation of FEF25%-75%.
Usually FEV1 is > 80% of FVC and the vital capacity should be > 80% of the predictive value. The predictive values will be dependent on gender, body size, and age. The total lung capacity (TLC) will be increased in the obstructive lung disease case but decreased if the diseases restricted. Normal maximum breathing capacity is about 125 l/min and the normal maximum midexpiratory flow rate is > 300 liter/min..
|
Pulmonary Parameter |
Obstructive Disease |
Restrictive Disease |
|
VC |
Normal or decreased |
Decreased |
|
TLC |
Normal or increased |
Decreased |
|
RV |
Increased |
Decreased |
|
FEV1/FVC |
Decreased |
Normal or increased |
|
MMEFR (FEF25%-75%) |
Decreased |
Normal |
|
MBC |
Decreased |
Normal |
VC: vital capacity, TLC total lung capacity, RV residual volume, MBC maximum breathing capacity
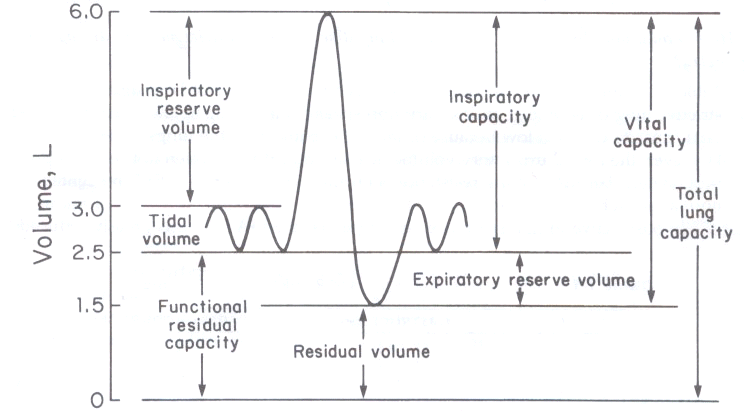
5Lung volume and capacity definitions
Tidal volume: volume of air inhales are exhaled during normal breathing (VT); normal values approximate 0.5L
Inspiratory reserve volume (IRV): maximum gas lion that can be inhaled following the normal inspiration at rest; normal values for IRV approximate 3 L
Expiratory reserve volume (ERV): maximal gas volume that can be exhaled following a normal expiration; normal values for ERV approximate 1 L.
Residual volume (RV) gas volume remaining in the lungs following forced exhalation; normal RV values approximate 1.5 L.
Vital capacity (VC) is the maximal gas amount they can be exhaled following maximal inhalation. While capacity (VC) equals VT + ERV + IRV; normal VC approximates 4.5L
Functional residual capacity (FRC) is the lung volume that remains following a normal quiet expiration. The value should be the sum of RV + ERV. Typical FRC values approximate 2.5 L.
Inspiratory capacity (IC) is the gas amount they can be inhaled from resting expiratory position following a normal exhalation and is the sum of VT + IRV. The normal IC value approximates 3.5 L.
Total lung capacity (TLC) is the lung volume following maximal inspiration. The TLC is the sum of VC and RVC. Normal TLC values approximate 6 L
 |
Flow volume loops characterize airflows as a function of volumes; that is, flows and flow volume are plotted concurrently.
The process is initiated by inspiration to total lung capacity followed by a forced vital capacity activity.
Maximal, rapid, inhalation is then performed back to total lung capacity.
The effort dependent part of the loop includes the entire inspiratory components and the expiratory part of the curve near the total lung capacity.
By contrast, the expiratory flow from about 25% to about 75% of the vital capacity is effort independent.
Usually the ratio of expiratory flow to inspiratory flow at 50% of vital capacity (mid-VC flow ratio) approximates 1.
This ratio is useful in the identification of upper airway obstruction.
Patients with pulmonary fibrosis or scoliosis ( restricted defects), exhibit a reduced FVC while retaining a comparatively normal FEV1.
Total lung capacity will be reduced while FEF 25%-75% and mid-VC flow ratio remains normal.
In patients with obstructive lung defect, peak expiratory flow rate, FEF25%-75% and mid-VC flow ratios will be reduced although total lung capacity (TLC) will be increased due to increases in RV.
|
|
|
|
|
An obstruction may be variable in that the magnitude of the effect depends on respiratory phase.
In the presence of tracheal stenosis (see above graph) or vocal cord paralysis, examples of variable extrathoracic obstructions, respiratory flow during forced inspiration will be reduced since the negative transmural pressure inside the airway tends to collapse the airway.
By contrast, expiratory flow will be reduced much less and could even be normal since positive pressure inside the airway will decrease the obstruction.
Variable intrathoracic obstruction results in reduced expiratory flow since high positive intrapleural pressures during forced expiration will decrease the airway diameter.
By contrast again, inspiratory flow will be much less reduced because negative intrapleural pressure will tend to increase airway diameter.
5,7Closing Capacity and Volume
Closing capacity (CC) is defined as the lung volume at which small airways in the dependent part of the lung close. Closing capacity = closing volume + residual volume. Closing volume is defined as the gas volume expelled during phase IV of the single-breath nitrogen test-reflecting the lung volume from the beginning of airway closure to the end of maximal expiration. Consequently, CV =CC - RV.
In normal, young individuals, the closing volume corresponds to about 10% of vital capacity or 0.4-0.5 L. Closing volumes and capacity to increase with age; also, closing volume increases in patients with small-airway disease and in individuals who smoke chronically.
|
|
|
Age and posture effects on functional residual capacity (FRC) and closing capacity (CC)
FRC appears only marginally sensitive to age, possibly increasing slightly.
Closing capacity increases with age, becoming equal to FRC at about age 66 (upright position) and about age 44 (supine position) and FRC increases about 30% when the individual changes from supine position to upright position.
Closing capacity is not dependent on body position.
In determination of whether airway closure has occurred, the effects of age on closing capacity and posture on FRC could be important.
Anesthesia effects on FRC & CC
FRC decreases about 20% with spontaneous breathing and about 16% with mechanical ventilation during anesthesia.
This effect is based on changes in thoracic cage muscle tone.
Following general anesthesia induction, reduction in inspiratory tone and appearance of end-expiratory tone in abdominal expiratory muscles at the end of exhalation occur.
End-expiratory tone in abdominal muscles increases intraabdominal pressure which moves the diaphragm cephalad thus decreasing FRC. Probably closing capacity declines in parallel with FRC during anesthesia.
Relationships between FRC and oxygenation
If FRC decreases below closing capacity, airways will close in the dependent lung regions during certain periods of normal tidal ventilation. Such airway closure causes pulmonary blood flow shunting through the unventilated alveoli. Therefore, QS/QT increases and arterial oxygenation decreases.
Pulmonary circulation and alveolar gas exchange occur all the time, during inspiration and expiration. Therefore, independent of airway closure, blood oxygenation during expiration will be depend primarily on remaining lung volume, or FRC.
When FRC is high, therefore, blood oxygenation will be relatively better since there is more time for oxygenation prior to hypoxemia developing during apnea. Finally, FRC is decreased in the supine position during general anesthesia as well as in adult respiratory distress syndrome.
Positive end-expiratory pressure (PEEP) has the effect of increasing FRC and decreasing airway closure.
5Lung compliance, chest wall compliance, and total compliance:
Lung compliance is defined as the change in lung volume per unit change in alveolar/intrathoracic pressure gradient. (Normal value approximates 200 ml/cm H2O in the upright position.)
Chest wall compliance is defined as the change in lung volume per unit change in ambient/intrathoracic pressure gradient. (Normal values for chest wall compliance approximate 200 ml/cm H2O.)
Total compliance will be the change in lung volume per unit change in alveolar/ambient pressure gradient giving in accord with the previous values, the normal value of about 100 ml/cm H2O; the equation is of the form:
1/lung compliance + 1/chest wall compliance = 1/Total compliance
|
5Analysis of the the following arterial blood gas data: pH, 7.36; pCO2, 60 mmHg; PO2, 70 mmHg, CO2 content, 36 mEq/L
FIO2 is needed to evaluate PaO2, If the blood were obtained while the patient was inspiring room air, then the data indicate respiratory acidosis, compensated by metabolic alkalosis and slight hypoxemia. These data would be consistent with chronic obstructive lung disease.
If FIO2 is 1.0, then normal PaO2 would be about 500 mmHg - 600 mmHg. Estimation of PaO2 at different values of FIO2, is made easier with the reasonable assumption that every 10% increase in O2 causes at 50 mmHg - 60 mmHg increase in PaO2. So, if the FIO2 is 0.4 then the normal PaO2 would be 200 mmHg - 240 mmHg.
5 Question: What would be the general preoperative workup orders:
Standard tests would include CBC, serum electrolytes, urinalysis, ECG, coagulation testing, with extravigilance paid to cardiopulmonary system assessment, chest x-ray, pulmonary function testing including determination of responsiveness to bronchodilators and determination of baseline arterial blood gas data. History would emphasize determination of possible allergies and present symptoms.
In patients with asthma, and particular preoperative considerations would include the following:
resolution of any acute or chronic infection by means of antibiotic treatment;
use of bronchodilators to relieve acute bronchial spasm;
chest physiotherapy to promote bronchial drainage and sputum clearance;
administration of diuretics, positive inotropic agents, oxygenation for management of uncompensated or borderline cor pulmonale;
correction of dehydration and electrolyte imbalances;
preoperative familiarization with any respiratory therapy equipment that could be used postoperatively;
efforts promoting patients' smoking cessatoin for at least two months prior to surgery with the aim of improving mucociliary clearance and reduction of sputum production;
in patients who continue to smoke, attempt to have the patient stop smoking for at least 12 hours prior to the procedure to reduce carboxyhemoglobin levels which should improve blood oxygen content promoting release of oxygen in hemoglobin;
used of cromolyn inhalation up to the time to surgery to reduce mast-cell degranulation with subsequent bronchoconstrictive mediator release;
5Question: Delay of surgery of patient has had recent upper respiratory infection?
As discussed previously, respiratory infections promote increased airway hyperreactivity and predisposed to acute asthma exacerbation. In both normal individuals and those with asthma, enhanced airway responsiveness may last from 2-6 weeks. Risk of respiratory complications in children who had experienced both recent upper respiratory tract infection and endotracheal anesthesia appears significantly elevated. Laryngospasm and bronchospasm incidence were increased in non asthmatic children 2 weeks after upper respiratory tract infections.
Probably waiting 2-3 weeks after clinical recovery from upper respiratory tract infections, even the absence of any clinical presentation, appears reasonable.
5Probable medications that asthmatic patient would be taking prior to surgery:
Bronchodilators are likely candidates including sympathomimetics and methylxanthine agents.
The patient may also have been taking systemic glucocorticoids, but more likely has been taking these agents by aerosol in an effort to minimize systemic side effects and promote more focused local therapy. Beta-2 selective adrenergic agonists such as albuterol, fenoterol, terbutaline, or bitolterol.are probably being administered. Anticholinergic bronchodilators may also be used, in an effort to reduce baseline parasympathetic tone, such as ipratropium bromide and methylatropine nitrate. Cromolyn (mass cell stabilizer) and aerosol steroids would also be likely medications.
5Question: rationale for ordering preoperative steroids?
Preoperative glucocorticoid administration would be reasonable for patients with suspected (or known) adrenal insufficiency. It is possible that patients who been chronically treated with high-dose glucocorticoids within the previous year may have some adrenocortical suppression.
Adrenal glands secretion corresponds to about 30 milligrams of hydrocortisone (cortisol) per day in normal circumstances; however, stress can induce 200-500 mg secretion. It is perhaps reasonable to replace 300 mg of cortisol per day during the preoperative time frame. The night preceding surgery 100 mg of hydrocortisone acetate might be provided intramuscularly with an additional 100 mg hydrocortisone those in an IV before induction and during operation.
Postoperatively, hydrocortisone phosphate, 100 mg IV, maybe given every eight hours for 48 hours with subsequent tapering; cortisol half-life is about 8-12 hours.
If the patient has < one week systemic steroid treatment or the treatment was over six months previously with no sign of adrenal insufficiency, routine steroid administration would not be appropriate.
Nevertheless, IV steroid preparations should be available in the OR should adrenal insufficiency-induced hypotension occur during the procedure.
In patients with moderate to severe asthma, it is possible that systemic steroids should be used preoperatively particularly if the patient required steroids previously.
Wound healing is not thought to be influenced by one day of high-steroid administration.
If ongoing wheezing is occurring and the surgery is elective, a week of steroid treatment might be appropriate. Onset of action of IV steroids may require many hours (6 or more in some circumstances). Following bronchodilators therapy (aggressive), unresolved severe bronchospasm may mandate IV corticosteroid administration. In this case, a loading dose of hydrocortisone at 4 mg/kg would be given to achieve a plasma cortisol level > 100 mcg/dl, followed by 3 mg/kg every 6 hours.
Question: should atropine or another anticholinergic be administered to the asthmatic patient?
Although anticholinergic (antimuscarinic) agents may be part of preoperative medication in some circumstances, their use in the asthmatic patient may be problematic because these agents causes mucus drying and may promote airway plugging.
Question: if the patient has a severe asthma attack in the OR any attack occurs before anesthesia induction, should the surgery be postponed or should the procedure continue?
Medical treatment would be initiated to manage the asthmatic attack; however, if the surgery were elective, it would be postponed.
Following postponement, the patient would be evaluated carefully and perhaps prepared more effectively before the next surgical attempt, in order to decrease the likelihood of a repeat asthma exacerbation. Should the surgery be emergent in nature, following resolution of the acute asthma episode, surgery may be continued along with continued medical intervention to insure management of the airway.
Question: suppose our patient did not have an asthma attack in the OR, what would be the options for anesthesia induction?
Three important principles applied to the most effective anesthetic management for the patient with asthma.
These are to
(1) attenuated airway reflexes before laryngoscopy and intubation
(2) relax airway smooth muscle and
(3) decrease the likelihood of bronchospasm mediator release.
Possibly methohexital could be used for induction followed by oxygen with a potent inhalational agents which could be halothane, enflurane, or isoflurane. These agents would be administered by mask until sufficient anesthesia depth would be obtained for tracheal intubation followed by succinylcholine administration.
Endotracheal spray with topical lidocaine (80 mg-120 mg) could be used before intubation to decrease the cough reflex, but as noted earlier, lidocaine may itself induce a cough reflex if the anesthesia depth is too light.
Question: why would methohexital be possibly more appropriate than thiopental?
Thiopental and thiamylal are probably more likely to cause histamine release than methohexital or pentobarbital. Possibly the sulfur atom present in the thiobarbituates is important in histamine release promotion. Methohexital, which does not contain the sulfur atom, may be less likely to cause histamine release which would promote bronchospasm particularly in the asthmatic patient. Thiopental or methohexital can be used successfully in the asthmatic patient provided the airway is not stimulated until inadequate anesthetic depth has been obtained.
Question: what about propofol, etomidate or ketamine as an agent for induction?
In the asthmatic patient, propofol might be the induction agent of choice providing the patient was hemodynamically stable. 2.5 mg/kg propofol seems to cause a reduced incidence of wheezing following into tracheal intubation compared with 5 mg/kg thiopental or thiamylal or 1.75 mg/kg methohexital. Propofol may be also associated with lower respiratory resistance following tracheal intubation compared to thiopental or etomidate. Etomidate does not decrease myocardial contractility which would be particularly important in patients in which hemodynamic stability is of critical concern. For patients with unstable hemodynamic status, ketamine, which produces bronchodilation, may be the agent of choice if the patient is actively wheezing.
Question: would lidocaine be reasonable to use for intubation?
Reflex-induced bronchospasm might be avoided by intravenous lidocaine (1 mg/kg) administered 1-2 minutes prior to endotracheal intubation. Topical spraying may be problematic because the spraying itself can cause reflex bronchoconstriction in the absence of sufficient anesthetic depth. Lidocaine infusion (1-2 mg/kg/hour) may be appropriate in cardiac or elderly patients with COPD because the patient's airway may need more anesthesia than may be tolerated by the cardiovascular system.
Question: if the circumstance requires emergency surgery with rapid-sequence induction, what procedure might be used in this patient?
In the emergent setting care must be taken to not only prevent an asthmatic attack but also to make less likely for gastric content aspiration to occur. Rapid sequence induction and tracheal intubation using propofol, thiopental, or methohexital and succinylcholine would probably prevent aspiration, yet precipitation of severe bronchospasm could occur in the absence of sufficiently deep anesthesia. In noncardiac asthmatic patients, ketamine (2 mg/kg) may be the agent of choice since it promotes catecholamine release which then causes bronchodilatation. However, if the asthmatic patient also has ischemic heart disease, fentanyl 5 mcg/kg, 2-3 minutes prior to methohexital administration (1.5 mg/kg) has the effect of airway reflex suppression, prevention of tachycardia and prevention of intubation-induced hypertension.
IV lidocaine (1-2 mg/kg) administered immediately before ketamine or fentanyl and succinylcholine is probably useful adjunct drug, helping to prevent reflex bronchospasm. This agent may be particularly important in emergency setting when deep anesthesia cannot be achieved prior to induction.
A full stomach should be empty using nasogastric tube suction. Denitrogenation should proceed using 100% mask oxygen. Pancuronium or vecuronium, 1 mg, should be given 3 minutes prior to succinylcholine administration.
Should the patient have a wheezing attack prior to anesthesia, a loading dose of aminophylline could be used to reduce bronchospasm followed by continuous IV aminophylline infusion. Sympathomimetic administration by inhalation (e.g. albuterol) is probably the first-line treatment for acute asthma attack.
Question: which muscle relaxants might be preferable in this case?
Agents should be chosen that are unlikely to provoke histamine release. These agents include rocuronium, cisatracurium, vecuronium and pancuronium. By contrast, d-tubocurarine can induce brochospasm by histamine release.
1Stoelting, RK and Dierdorf, SF, "Asthma" in Anesthesia and Co-Existing Disease, 4th edition, Chapter 14, pp 194-204, Churchill-Livingstone, Philadelphia, 2002
2Kingston, HGG, Hirshman, CA Perioperative management of the patient with asthma. Anesth Analg 1984; 63: 844-55. (second sourced from reference 1)
3Roisen, MF, "Anesthetic Implications of Concurrent Diseases" in Anesthesia, 5th edition, (Miller, R.D, editor), Chapter 25, pp 996-997, Churchill-Livingstone, Philadelphia 2000
4Que, JC and Lusaya, VO Sevoflurane Induction for Emergency Cesarean Section in a Parturient in Status Asthmaticus, Anesthesiology, 1999; 90: 1475-1476.
5Yao, F-S F, "Asthma-Chronic Obstructive Pulmonary Disease (COPY) in Yao and Artusio'sAnesthesiology: Problem-Oriented Patient Management, 4th ed, Yao, F-S. F, editor, Chpater 1, pp 3-28, Lippincott Williams & Wilkins, Philadelphia
6Goudsouzian, N and Karamanian, A: Physiology for the Anesthesiologist, 2nd ed. Norwalk, CT, Appleton-Century-Crofts, 1984 (second sourced from ref. 5)
7Respiratory Care: Midland College
8Elton, D.R. " Shunt and Deadspace: 1990
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |