|
|
Medical Pharmacology Chapter 29: Diabetes
Insulin binds to target receptors (high affinity, high specificity) and liver, muscle, and fat tissue
Two heterodimers
Each containing an alpha subunit (extracellular: recognition site) and
A beta subunit which spans the membrane and contains a tyrosine kinase.
|
|
|
![]() The
insulin receptor (IR) belongs to the tyrosine kinase receptor
class and is activated by insulin, IGF-I and IGF-II
(insulin-like growth factor I and insulin-like growth factor
II).
The
insulin receptor (IR) belongs to the tyrosine kinase receptor
class and is activated by insulin, IGF-I and IGF-II
(insulin-like growth factor I and insulin-like growth factor
II).
|
|
|
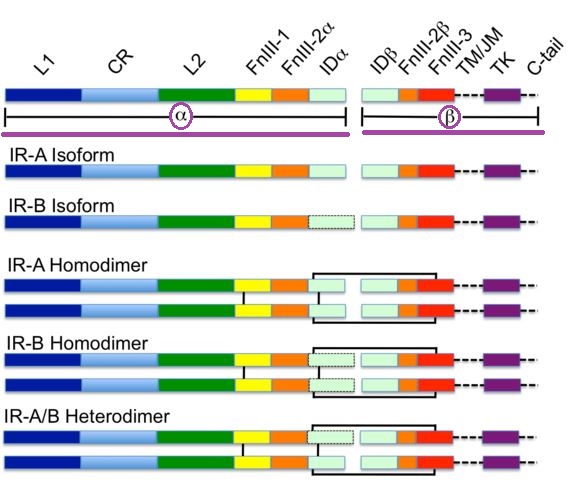
The receptor is encoded by a single gene (INSR) and during transcription alternate splicing results in either IR-A or IR-B isoforms.
Downstream from this step these isoforms undergo reactions that form proteolytically cleaved α and β subunits.
The subunits combine to form either homodimers or heterodimers, resulting in the disulfide-linked transmembrane insulin receptor.
Attribution: Belifore A Frasca F "Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hydrids in physiology and disease" Endocr Rev 30(6): 586-623.
 |
|
|
|
|
Insulin binds to alpha subunit
Beta subunit increases tyrosine kinase activity, resulting in auto- phosphorylation
Phosphorylated beta subunit promotes aggregation of heterodimers and stabilizes the receptor tyrosine kinase activated state
Docking protein (insulin receptor substrate-1, IRS-1) is then phosphorylated
Phosphorylated IRS-1 activates other kinases, promoting further phosphorylation reactions
Insulin's second messengers: these phosphorylation products
Consequence: glucose transporter translocation from sequestered sites to exposure on the cell surface
Insulin-receptor complex is then internalized
Alteration in Insulin receptor affinity
Decrease affinity caused by some hormonal agents (e.g. hydrocortisone).
Increase affinity caused by excess growth hormone.
Karam, J. H., Pancreatic Hormones and Antidiabetic Drugs, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 684-703
Foster, D. W., Diabetes Mellitus, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 2060-2080
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |