|
|
|
-
-
Drug Clearance
-
Attribution:
-
Saffarzadeh A Clearance and Rate of Elimination - Pharmacokinetics
-
Areo Saffarzadeh Youtube Channel
-
-
-
Introduction
-
Clearance is especially important for insuring appropriate long-term drug dosing with correct steady-state drug concentrations
. -
Clearance of a given drug is usually constant over the therapeutic concentration range because:
-
Drug elimination systems are not saturated.
-
Because the elimination system is note saturated, the absolute rate of elimination is a linear function of the drug plasma concentration.
-
 In this case, drug elimination is
therefore usually a first-order kinetic
process indicating that a constant fraction of the drug
is eliminated per unit time.
In this case, drug elimination is
therefore usually a first-order kinetic
process indicating that a constant fraction of the drug
is eliminated per unit time.
-
-
 If the elimination system is
saturated zero order kinetics apply and a
constant amount of drug is eliminated
per unit time. (Clearance is variable)
If the elimination system is
saturated zero order kinetics apply and a
constant amount of drug is eliminated
per unit time. (Clearance is variable)-
Ethanol clearance follows zero order kinetics, as an example.
-
-
-
Clearance: the drug's rate of elimination (by all routes) normalized to the concentration of drug C in some biological fluid:
-
CL = Rate of elimination / C
-
CL = Vd x kel where Vd = volume of distribution and kel is the elimination rate constant
-
CL = Vd x (0.693/t1/2) where 0.693 = ln2 and t1/2 is the drug elimination half-life.
-
-
-
Clearance:
-
Volume per unit time (volume of fluid i.e. blood or plasma that would be completely freed of drug to account for the elimination)
-
May be defined as:
-
Blood clearance, CLb
-
Plasma clearance, CLp
-
Concentration of unbound or free drug, depending on the concentration measured (Cb, Cp or Cu)
-
-
-
Clearance is additive: a function of elimination by all participating organs such as liver or kidney:
-
CL systemic = CLrenal + CLhepatic + CLother
-
"Other" sites may include the lungs and other sites of drug metabolism (muscle, blood)
-
 The two most
important sites for drug
elimination: kidneys and liver.
The two most
important sites for drug
elimination: kidneys and liver.-
Renal clearance: clearance of unchanged drug and metabolites
-
Nephron Physiology
-
Attribution: Madhero88 / CC BY (https://creativecommons.org/licenses/by/3.0)
-
https://commons.wikimedia.org/wiki/File:Physiology_of_Nephron.png
-
-
Kidneys: most important organs for unchanged drug/drug metabolites elimination
-
Water-soluble compounds exhibit more efficient renal excretion compared to lipid soluble compounds (emphasizing the importance of metabolic conversion of lipid-soluble drugs to water-soluble metabolites)
-
Renal drug clearance is correlated with exogenous creatinine clearance or serum creatinine concentration
-
 Factors in renal
excretion:
Factors in renal
excretion:-
Glomerular filtration important considerations:
-
Fraction of free drug (compared to protein-bound drug)--when a drug is bound to protein it is not filtered
-
Glomerular filtration rate
-
-
Tubular secretion (active process)
-
Drug/metabolite selectivity is important.
-
-
Passive tubular reabsorption with important considerations including:
-
Enhanced lipid solubility favors reabsorption (lipid-soluble agents more readily cross renal tubular epithelial cell membrane thus entering pericapillary fluid)
-
Example: thiopental (highly lipid-soluble): completely reabsorbed -- minimal unchanged drug excreted in urine
-
 Renal
tubular reabsorption rate
influenced by:
Renal
tubular reabsorption rate
influenced by:-
pH
-
Rate of renal tubular urine flow
-
Weak acid or weak base drug/drug metabolite pKa compared to urinary pH
-
-
-
-
-
 Hepatic
clearance: drug elimination following
metabolic transformation of the parent
drug to metabolites
Hepatic
clearance: drug elimination following
metabolic transformation of the parent
drug to metabolites-
Since elimination is not "saturable", elimination is typically first order and directly proportional to drug concentration:
-
Rate of elimination = CL x C
-
-
-
-
-
-
 Other factors affecting renal
clearance
Other factors affecting renal
clearance-
Renal disease
-
Rates of filtration depend on:
-
Volume filtered in the glomerulus
-
Unbound drug concentration in plasma (plasma protein-bound drug is not filtered)
-
-
Drug secretion rates:
-
Extent of drug-plasma protein binding
-
Carrier saturation
-
Drug transfer rates across tubular membranes
-
Rate of drug delivery to secretory sites
-
-
Changes in plasma protein concentration
-
Blood flow
-
Number of functional nephrons
-
-
 Factors affecting hepatic clearance:
Factors affecting hepatic clearance:-
Drug delivery to hepatic elimination sites may be rate-limiting for certain drugs:
-
Also called flow dependent elimination: in this case most of the drug in the blood is eliminated on the first pass of the drug through the organ
-
Ahese drugs are termed "high-extraction"
-
-
Extent of plasma protein-bound drug
-
Blood flow (affects clearance on drugs with high extraction ratios).
-
Some drugs with clearances > 6 ml/min/kg
-
Chlorpromazine: (antipsychotic)
-
Diltiazem: (Ca2+ channel blocker)
-
Imipramine: (tricyclic antidepressant)
-
Lidocaine: (antiarrhythmic)
-
Morphine: (opioid analgesic)
-
Propoxyphene: (opioid analgesic)
-
Propranolol: (beta adrenergic receptor blocker)
-
Verapamil: (Ca2+ channel blocker)
-
Meperidine: (opioid analgesic)
-
Desipramine: (tricyclic antidepressant)
-
Amitriptyline: (tricyclic antidepressant)
-
Isoniazid: (anti-tuberculosis)
-
-
Changes in the intrinsic clearance (i.e. enzyme induction, hepatic disease: affects clearance of drugs with low extraction ratios): Examples --
Social factors:
Tobacco smoke induces some hepatic microsomal drug metabolizing enzyme isoforms (CYP1A1, CYP1A2, and possibly CYP2E1)
Chronic ethanol use induces CYP2E1
Dietary considerations:
Grapefruit juice contains chemicals that are potent inhibitors of CYP3A4 localized in the intestinal wall mucosa
Cruciferous vegetables such as brussels sprouts, cabbage, cauliflower and hydrocarbons present in charcoal-broiled meats can induce CYP1A2.
Calcium present in dairy products can chelate drugs including commonly used tetracyclines and fluoroquinone antibiotics.
Age: Neonates have reduced hepatic metabolism and renal excretion due to relative organ immaturity. On the other hand, elderly patients exhibit differences in absorption, hepatic metabolism, renal clearance and volume of distribution.
Genetic Factors:
Genetic polymorphism affecting CYP2D6, CYP2C19, CYP2A6, CYP2C9, and N-acetyltransferase
These changes may result in significant inter-individual differences in drug-metabolizing abilities (the drug of course must be a substrate for one of the above cytochrome P450 isoforms)
Certain genetic polymorphisms are associated with ethic groups.
For instance, 5%-10% of Caucasians are poor metabolizers of CYP2D6 substrates.
By contrast, the frequency in Asian populations is about 1%-2%. On the other hand, the incidence of poor metabolizers of CYP2C19 drugs is about 20% in Asian populations, but only about 4% in Caucasian populations.
Definition: genetic polymorphism -- "Genetic polymorphism is a type of variation in which individuals was sharply distinct qualities co-exist as normal members of the population" Ford, 1940.
Cytochrome P450 isoform naming conventions:
Review: drug biotransformation usually involves two phases, phase I and phase II.
Phase I reactions are classified typically as oxidations, reductions, or hydrolysis of the parent drug. Following phase I reactions, the metabolites are typically more polar (hydrophilic) which increases the likelihood of their excretion by the kidney. Phase I metabolic products may be further metabolized
Phase II reactions often use phase I metabolites can catalyze the addition of other groups, e.g. acetate, glucuronate, sulfate or glycine to the polar groups present on the intermediate. Following phase II reactions, the resultant metabolite is typically more readily excreted.
Most phase I reactions are catalyzed by the cytochrome P450 system (CYP).
This superfamily consists of heme-containing isoenzymes which are mainly localized in hepatocytes, specifically within the membranes of the smooth endoplasmic reticulum.
The primary extrahepatic site containing CYP isoforms would be enterocytes of the small intestine.
The gene family name is specified by an Arabic numeral, e.g. CYP3. > 40% of sequence homology characterize CYP isoforms within a family.
CYP families are subdivided into subfamilies designated by an upper case letter, it e.g. CYP3A .
Gene numbers of individual enzymes are noted by a second Arabic numeral following the subfamily letter, e.g. CYP3A4.
CYP isoforms not only metabolize many endogenous substances including prostaglandins, lipids, fatty acids, and steroid hormones but also metabolize (detoxify) exogenous substances including drugs
Major CYP isoforms responsible for drug metabolism include: CYP3A4, CYP2D6, CYP2C9, CYP2C19, CYP1A2, CYP2E1 in in certain cases CYP2A6 and CYP2D6
Important enzymes for phase II reactions include:
Glutathione-S-transferases
UDP-glucuronosyl transferases
Sulfotransferases
N-acetyltransferases
Methyltransferases and
Acyltransferases.
Zero Order (and first order) Elimination
Saffarzadeh A First Order and Zero Order Elimination https://www.youtube.com/watch?v=XEotDfKhNTw . (10/2012)
Areo Saffarzadeh Youtube Channel
Capacity-limited elimination (zero-order):
Drug examples: ethanol, aspirin.
Capacity-limited elimination:
Saturable, dose-or concentration-dependent
Nonlinear
Michaelis-Menten elimination
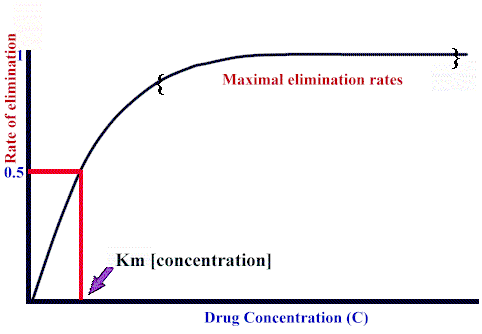
If blood flow to the organ does not limit elimination, the relationship between the elimination rate and drug concentration,C, is:
Rate of elimination = Vmax · C / (Km + C)
the form of this equation is very similar to the Michaelis-Menten description of enzyme kinetics. Here, however:
Vmax refers to maximum elimination capacity
Km is the drug concentration at which the rate of elimination is 50% of Vmax.
As expected from the rectangular-hyperbolic shape of the curve, at high drug concentrations (compared to the Km), dependency of elimination rate on drug concentration decreases significantly, approximating zero order behavior. see below:
Holford, N. H.G. and Benet, L.Z. Pharmacokinetics and Pharmacodynamics: Dose Selection and the Time Course of Drug Action, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 34-49.
Benet, Leslie Z, Kroetz, Deanna L. and Sheiner, Lewis B The Dynamics of Drug Absorption, Distribution and Elimination. In, Goodman and Gillman's The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) The McGraw-Hill Companies, Inc.,1996, pp. 3-27
Stoelting, R.K., "Pharmacokinetics and Pharmacodynamics of Injected and Inhaled Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 1-17.
![]()
-
Overview of Drug Half-Life
-
Saffarzadeh A Drug Half-life https://www.youtube.com/watch?v=eTqPsqnbwoc . (10/2012)
-
Areo Saffarzadeh Youtube Channel
-
-
Introduction
-
 Half-life: (t1/2)
represents the time required to decrease the amount
of drug in body by 1/2 during elimination
(or during a constant infusion).
Half-life: (t1/2)
represents the time required to decrease the amount
of drug in body by 1/2 during elimination
(or during a constant infusion). -
Assumption:
-
Single body compartment size = volume of distribution (Vd)
-
Blood or plasma considered in equilibrium with total volume of distribution
-
-
t1/2 = (0.693 · Vd)/CL
-
t1/2 = (0.693)/kel
-
0.693 equals the natural logarithm of two. (Since drug elimination is an exponential process, the time required for a twofold decreased is proportional to ln(2)).
-
kel = km + kex; where the elimination rate, kel ,constant is the sum of the rate constants due to metabolism, km , and excretion,kex.
-
-
Factors affecting t1/2:
-
 Disease
states may affect volume of distribution
and clearance
Disease
states may affect volume of distribution
and clearance-
Example 1: a patient with chronic renal failure:
-
Decreased digoxin (Lanoxin, Lanoxicaps) renal clearance
-
Decreased Vd due to decreased renal and skeletal muscle mass (decreased digoxin tissue binding)
-
Resultant increase in digoxin half-life less than expected based on renal function change
-
-
Example 2: half-life of diazepam (Valium) increases with age;
-
Clearance does not change
-
Volume of distribution changes
-
-
Example 3: half-life changes secondary to changes in plasma protein binding.
-
Patients with acute viral hepatitis: half-life of Tolbutamide (Orinase) decreases (opposite of expected?)
-
Acute viral hepatitis alters plasma and tissue drug-protein binding; the disease does not change volume of distribution but increases total clearance because more free drug (not bound to protein) is present.
-
-
-
-
-
 Elimination halftime and anesthesia:
Elimination halftime and anesthesia:
-
Elimination halftime is important in estimating recovery from anesthetic drug administration.
-
In the case of IV administered agents, an inconsistency between the elimination halftimes following a single, bolus injection compared to continuous IV infusion, has resulted in the development of an idea of referred to as "context-sensitive or dependent" halftimes.
-
The definition of "context-sensitive" halftimes is the length of time required for the drug plasma concentration to fall 50% after continuous infusion
-
For IV anesthetic drug pharmacokinetics, special problems exist because those significant differences in individual drug requirements (up to 2-5 times) as a result of dose-plasma and plasma-effect relationships
-
-
By contrast to the above special problems associated with IV anesthetic drug pharmacokinetics and variation between drugs, a similar problem does not exist for the volatile agents were drug-effect relationships appear more predictable.
-
-
Half-life:
-
Useful in estimating time to steady-state: approximately 4 half-lives are required to reach about 94% of a new steady-state
-
Useful in estimating time required for drug removal from the body
-
Means for estimation of appropriate dosing interval
-
-
-
 With repeating drug doses,
the drug will accumulate in the body until dosing
ceases.
With repeating drug doses,
the drug will accumulate in the body until dosing
ceases.-
Practically: accumulation will be observed if the dosing interval is less than 4 half-lives.
-
Accumulation: inversely proportional to the fraction of the dose lost in each dosing interval
-
Accumulation factor = 1/Fraction lost in one dosing interval = 1/(1 - fraction remaining)
-
For example, the accumulation factor for a drug given once every half-life: 1/0.5 equals 2.
-
-
Stoelting, R.K., "Pharmacokinetics and Pharmacodynamics of Injected and Inhaled Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 1-17.
Dolin, S. J. "Drugs and pharmacology" in Total Intravenous Anesthesia, pp. 13-35 (Nicholas L. Padfield, ed), Butterworth Heinemann, Oxford, 2000