Medical Pharmacology Chapter 41: Anti-inflammatory Agents
|
|
|
Indole derivative
Well absorbed (oral route)
Substantial plasma protein binding
Hepatic metabolism
Inactive metabolites and unchanged parent compound: excreted in bile and urine
Not recommended for analgesia
Not recommended for use in children, except:
Treatment of patent ductus arteriosus in premature infants
Prostaglandin production keeps the structure open; accelerated closure, in a premature newborn can be accomplished by intravenous infusion of indomethacin
May avoid surgery
Renal toxicity may occur
COX-I dependent
Effective in clinical management of:
Acute gouty arthritis
May replace colchicine in initial treatment
Ankylosing spondylitis
Extra-articular inflammation, e.g.:
Pericarditis
Pleurisy
Bartter's syndrome
Controversial Use: tocolytic in preterm labor < 32 weeks gestation
Rationale: reduced prostaglandin synthesis ® reduced strength/frequency all of uterine contractions
Difficulty: fetal/maternal drug toxicity
Alternative Medication: calcium channel blockers
At higher dosages: about 33% have reactions sufficient to require withdrawal of indomethacin
Gastrointestinal disturbances:
Abdominal pain
GI hemorrhage
Pancreatitis
Diarrhea
Headache common: frequency = 15%-25% (including dizziness, depression, confusion)
Hyperkalemia, secondary to renal prostaglandin synthesis inhibition
Contraindicated in patients with:
Nasal polyps
Angioedema
Asthma
Pregnancy
Cautious Use: -- peptic ulcer disease; psychiatric disorder
Pyrazolone derivative
Hematologic toxicities: ® withdrawal from North American markets
Agranulocytosis/aplastic anemia ® deaths
Hemolytic anemia
Many other non-hematologic toxicities
Intermediate duration NSAID
Marketed for analgesia; not for inflammation (but has typical NSAIDs' properties)
Significant analgesic effects --sufficient to replace morphine in mild/moderate post surgical pain
Parenteral administration route most common
Ophthalmic preparation available
Clinical Pharmacological Issues:NSAIDs
NSAIDs:less gastric irritation; easier dosing schedule, e.g. better patient compliance
NSAIDs: safer than aspirin, more expensive
NSAIDs: gastric ulceration patients taking anti-inflammatory doses --
Approaches to reduce gastric irritation leading to ulceration:
Concurrent administration of misoprostol
Misoprostol (prostaglandin E1 analog) reduces (a) gastric acid secretion and (b) the increases gastric mucosal protective factors, e.g. bicarbonate
NSAIDs: increased incidence of acute renal failure; nephrotic syndrome
Insidious development
Not dose-dependent; not related to drug use duration
NSAIDs -- Prescribing Decisions:
Cost
Adverse effects
Dosing schedules-- patient compliance issues
Once a day/twice a day dosing:
Piroxicam
Naproxen
Sulindac
Oxaprozin
For patients taking hypoglycemic agents or warfarin:
Ibuprofen--no potentiation of hypoglycemic/warfarin effects
Tolmetin--no potentiation of hypoglycemic/warfarin effects
Rheumatoid arthritis:(DMARDS)
Overview:
Chronic multisystem disease
Unknown cause
Characteristic feature:
Persistent inflammatory synovitis, usually involving peripheral joints (symmetric distribution)
Consequences of synovial inflammation:Disease Hallmarks--
Cartilage destruction
Bone erosion
Joint integrity change
Range of Disease Manifestations:
Mild (brief, oligoarticular illness; minimal joint damage)
Substantial (progressive, relentless, polyarthritis ®significant functional impairment)
Prevalence of rheumatoid arthritis: 0.8%
women: affected about 3X more often than men
Prevalence increases with age
RA:
Affects all races
Seen worldwide
Most frequent onset: age--forties to fifties
Genetic Predispositions:
Severe RA: four times expected rate in first-degree relatives of individuals with disease associated with auto antibody, rheumatoid factor
About 10% of patient with RA -- affected first-degree relative
Monozygotic twins: at least four times more likely to be concordant for RA than dizygotic twins
About 15% to 20% of monozygotic twins -- concordant for RA suggesting other than genetic factors play an important role
Genetic Factors-- RA etiology and other considerations:
Class II major histocompatibility complex (MHC) gene product HLA-DR4
Approximately 70% of patients with definitive RA express HLA-DR4 (compared to 28% of controls)
Genes outside HLA complex contributes, probably including genes controlling T cell antigen receptor expression and the expression of immunoglobulin heavy and light chains
Genetic component associated with drug-induced toxicities -- For example:
presence of the HLA-DR3 (DRß1*0301) allele: highly correlated with side effect development to gold therapy including:
Proteinuria (similar relationship between allele presence and proteinuria associated with D-penicillamine treatment)
Skin rash
Thrombocytopenia
Non-genetic factors:
Analysis of epidemiologic studies in Africa;indicative of additional factors including
Climate
Urbanization
Pathology and pathogenesis
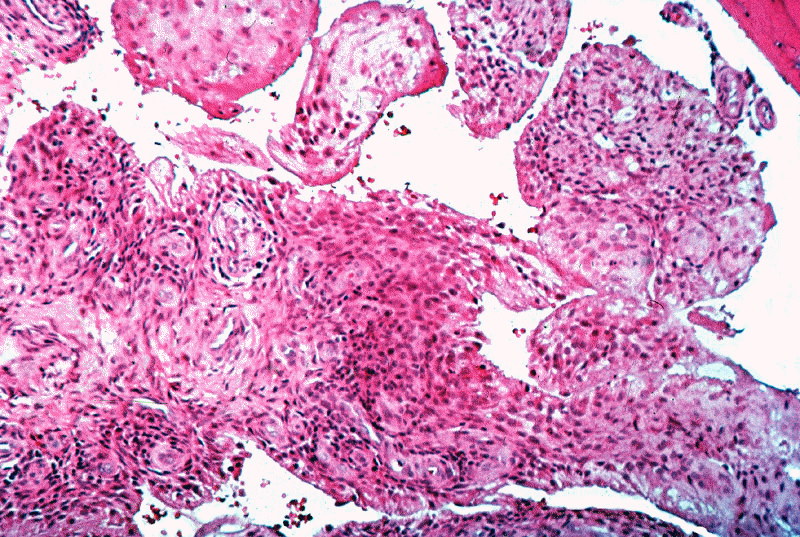
Earliest lesions in rheumatoid synovitis:
Increased number of synovial lining cells
Subsequent changes:
Increased number of synovial lining cells + mononuclear cell perivascular infiltration
Synovium: edematous; protruding into the joint cavity
 |
|
Microscopic features:
Synovial lining cell hyperplasia and hypertrophy
Focal/segmental vascular change --
Microvascular injury
Thrombosis
Neovascularization
Edema
Mononuclear cell infiltration (aggregates around small blood vessels)
Rheumatoid synovium endothelial cells:
Resemble high endothelial venules of lymphoid organs
Altered by cytokine exposure
elaboration of adhesion molecules
Infiltrating cell types:
Predominant -- T lymphocytes
CD4+ memory T cells more common then CD8+ cells
T cell's express early activation antigen CD69
Significant B-cell proliferation:
local differentiation into antibody-producing plasma cells
produce both polyclonal immunoglobulin and autoantibody rheumatoid factor ® local immune complexes formation.
HLA-DR+ macrophages
Dendritic cells
Synovial fibroblasts are activated producing: collagenase/cathepsins ® articular matrix degradation
Activated fiberglass: prominent:
Lining layer
Bone/cartilage interface -- osteoclasts prominent
At bone erosion sites
Rheumatoid synovium:
Secreted products of activated lymphocytes, macrophages, fibroblasts
Local cytokine/chemokine production responsible for many clinical and pathological presentations of rheumatoid arthritis
Rheumatoid Arthritis: Clinical Manifestations
Onset:two-thirds of patients -- following symptoms:
Fatigue, anorexia, weakness, vague musculoskeletal symptoms
Specific symptoms appear gradually:
symmetrical effects on joints of the hands, wrists, knees, feet
Symptoms of Articular disease
Most common manifestation of established RA:
pain and affected joints
worsened by movement
morning stiffness
Synovial inflammation: swelling, tenderness, motion limitations.
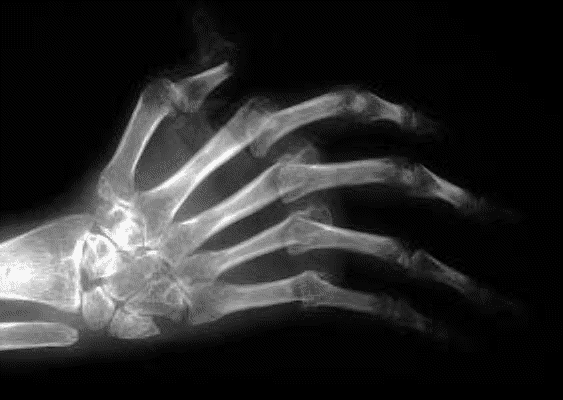
With persistent inflammation: characteristic deformities:
 |
|
Reduces mobility
Reduces quality of life
Reduces life span
NSAIDs: symptomatic relief (reduction: inflammation, pain) -- limited effect on progressive bone and cartilage loss
Disease modifying antirheumatic drugs (selecting -- six weeks to six months for effects) slow or possibly stop disease progression.
|
Methotrexate |
Azathioprine |
Penicillamine |
Hydroxycholoroquine |
|
Chloroquine |
Organic gold compounds |
Sulfasalazine |
Immunosuppressive drugs (DMARDs): useful in:
lupus nephritis
seropositive, progressive rheumatoid arthritis
vasculitis syndromes (Wegener's gramulomatosis and panarteritis)
Toxicities associated with immunosuppressive drug use (suggest safer agents should be prescribed first):
Oncogenic effects
Bone marrow depression
Hepatoxicity
Methotrexate: as a disease modifying antirheumatic drug
Mechanisms of action (rheumatic disease doses):
Inhibition of aminoimidazolecarboxamide ribonucleotide (AICAR) transformylase, thymidylate synthase and increased adenosine release.
70% absorbed (oral route administration)
Primary excretion pathway: urine; secondary, bile
Most common toxicities: Methotrexate
Mucosal ulcers
Nausea
Dose-related hepatotoxicity (enzyme changes): common
Reduction in methotrexate toxicity:
Post-treatment (24 hours after methotrexate): with leucovorin or using daily folic acid
Other immunosuppressive drugs that had been used for treating arthritis:
Mechlorethamine
Cyclophosphamide
Chlorambucil
Azathioprine, a purine antagonist, is FDA-approved for rheumatoid arthritis infrequent use due to toxicities: hematologic, hepatic, possible increased incidence of non-Hodgkin's lymphoma).
Primary Reference: Katzung, B. G. and Furst, D. E. Nonsteroidal Anti-Inflammatory Drugs; Disease-Modifying Antirheumatic Drugs; Nonopioid Analgesics; Drugs Used in Gout, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 578-602.
Lipsky, P.E. Rheumatoid Arthritis, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 1880-1888.Agudelo, C.A.
Gout in Medicine for the Practicing Physician, Fourth edition, (Hurst, J. Willis, editor in chief) Appleton and Lange, 1996, pp 223-226.
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |