|
|
|
Medical Pharmacology Chapter 33-34: Anticancer Drugs
Antimetabolites
Pyrimidine Analogues continued
Capecitabine (Xeloda)
![]() 5-fluorouracil
(Adrucil) may be administered directly as 5-FU or can be made available by
administration of the prodrug, capecitabine (Xeloda).1
5-fluorouracil
(Adrucil) may be administered directly as 5-FU or can be made available by
administration of the prodrug, capecitabine (Xeloda).1
Capecitabine is converted to 5-FU.
|
|
|
![]() Capecitabine (Xeloda)
is a fluoropyrimidine carbamate the may be taken orally.6
Capecitabine (Xeloda)
is a fluoropyrimidine carbamate the may be taken orally.6
Following significant and rapid gut mucosal absorption, capecitabine is associated with high oral bioavailability (about 80%).6
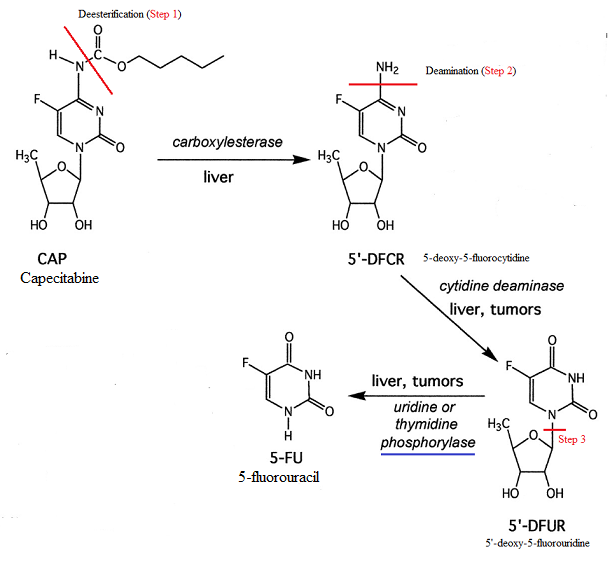
The parent form of the drug is inactive in requires 3 subsequent activating step facilitated by enzyme catalysis.
The last step (the 3rd step) occurs in tumor tissue thus allowing selective 5-FU activation in the target tissue.
This 3rd step is the conversion of 5'-deoxy-5-fluorouridine (5'-dFdU) to 5-fluorouracil (5-FU), a step catalyzed by thymidine phosphorylase (TP).
This enzyme (TP) is found at notably higher levels in tumors, compared to corresponding normal tissue.6
The two preceding steps involves deesterification (1st step) followed by deamination (2nd step).1
 |
|
![]() Capecitabine (Xeloda)
was initially introduced and approved for breast cancer shown anthracycline-and
taxane-resistant.6
Capecitabine (Xeloda)
was initially introduced and approved for breast cancer shown anthracycline-and
taxane-resistant.6
In a combination protocol with docetaxel, capecitabine was approved as second-line treatment for metastatic breast cancer.6
Capecitabine in combination with lapatinib (Tykerb),may be an appropriate option for treating HER2-positive metastatic breast cancer, if disease progression had been noted on trastuzumab (Herceptin)-based protocols.6
Lopatinib (Tykerb) is a tyrosine-kinase inhibitor of human epidermal growth factor receptor type II (HER2) and epidermal growth factor receptor (EGFR).
Capecitabine is also approved for:
First-line treatment of metastatic colorectal cancer (mCRC)6 and as
Adjuvant treatment for stage III colon cancer for those cases in which fluoropyrimidine monotherapy is desirable.6
In many countries, capecitabine in combination with oxaliplatin (Xelox) is viewed appropriate for treating mCRC and as adjuvant treatment in stage III colon cancer.
Absorption, Distribution, Biotransformation, Excretion:
Usually, capecitabine is given orally in two divided doses with food for 2 weeks followed by a one-week rest period.1
As noted earlier capecitabine is well absorbed following oral administration (about 80%).
Following rapid deesterification and deamination (see chart above) a high plasma level of an inactive prodrug 5'-dFdU is noted, exhibiting a t1/2 of about one hour.1
Thymidine phosphorylase catalyzes conversion of 5'-dFdU to the active drug, 5-FU with conversion occurring in peripheral tissues, liver, and tumors.
|
|
|
Liver dysfunction may delay conversion of the parent compound (capecitabine) to the prodrug 5'-dFdU and 5-FU, although no reproducible effect on toxicity has been reported.1
Excretion: Capecitabine and its metabolites are excreted mainly by the kidneys.
As a consequence, renal dysfunction interfering with drug excretion may require dosage adjustment.
![]() In patients
exhibiting creatinine clearance <30 mL/min, the use of capecitabine is
contraindicated.
In patients
exhibiting creatinine clearance <30 mL/min, the use of capecitabine is
contraindicated.
![]() There is, in
addition, an important drug-drug interaction that has been identified
between warfarin and capecitabine-based anticancer therapy.
There is, in
addition, an important drug-drug interaction that has been identified
between warfarin and capecitabine-based anticancer therapy.
Weekly monitoring of coagulation parameters for patients receiving warfarin and capecitabine concurrently is recommended along with warfarin dose adjustments as appropriate.
![]() Toxicity:3
Toxicity:3
The primary toxicities of capecitabine include:3
Diarrhea along with
Hand-foot syndrome (HFS).
![]() Myelosuppression, mucositis, alopecia, and nausea and vomiting although
observed following capecitabine administration occur less frequently than
following intravenous 5-FU administration.3
Myelosuppression, mucositis, alopecia, and nausea and vomiting although
observed following capecitabine administration occur less frequently than
following intravenous 5-FU administration.3
![]() Capecitabine
(5-FU pro-drug) has been approved for treatment of several cancers
including:1
Capecitabine
(5-FU pro-drug) has been approved for treatment of several cancers
including:1
Metastatic breast cancer not responsive to a protocol including paclitaxel and an anthracycline
Metastatic breast cancer when used along with docetaxel in those patients who have previously received an anthracycline-containing protocol and for
Metastatic colorectal cancer1
|
![]() Capecitabine has
been frequently used in combination with other drugs.1
Capecitabine has
been frequently used in combination with other drugs.1
One example is a combination with cisplatin for treating head and neck cancer.
Another example is combination of 5-FU and oxaliplatin or irinotecan for treating metastatic colorectal cancer (first-line treatment).1
![]() The use of 5-FU
in combination protocols confers improved survival as adjuvant breast cancer
treatment and similarly in combination with oxaliplatin and leucovorin in
colorectal cancer.
The use of 5-FU
in combination protocols confers improved survival as adjuvant breast cancer
treatment and similarly in combination with oxaliplatin and leucovorin in
colorectal cancer.
5-FU is an important and significant radiation sensitizer.
Beneficial effects have been noted when 5-FU is combined with radiation in primary patient treatment with:
Locally advanced esophageal cancer
Stomach cancer
Pancreatic cancer
Head and neck cancer
Cervical cancer and
Anal cancer 1
5-FU is also effective topically in treating premalignant skin keratoses and in treating multiple superficial basal cell carcinomas.1
Side effects associated with capecitabine administration are similar to those described for 5-FU infusion.
Major side effects include diarrhea and hand-foot syndrome (HFS).6
Likelihood of certain side effects including myelosuppression, mucositis, neutropenic fever, alopecia as well as nausea/vomiting is reduced with capecitabine compared to 5-FU.6
![]() Capecitabine
in addition to 5-FU and tegafur administration has been associated with a
relatively high rate (20%-50%) of alimentary tract mucositis.10
Capecitabine
in addition to 5-FU and tegafur administration has been associated with a
relatively high rate (20%-50%) of alimentary tract mucositis.10