Nursing Pharmacology: Antiviral Drugs
Antiviral Drugs
Anti-viral drugs with activity against HIV (Human Immunodeficiency Virus)
HIV-1 Pathophysiology/Pathogenesis
Long-term Infection: Latency Phase
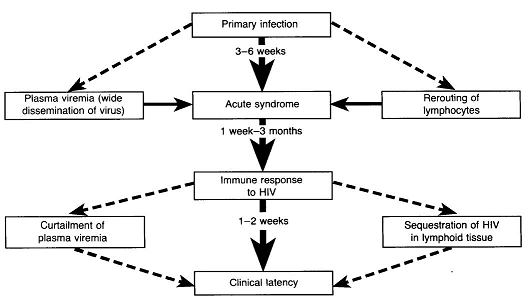
After the primary infection there is about a one week period before an initial viremia, which lasts 2-3 months.8
In many patients an acute mononucleosis-like presentation develops about a month following initial infection.
 |
|
About 50-70% of individuals with HIV infection progress through an acute clinical syndrome about a month following primary infection. (See figure above).2
Despite variability in clinical severity during this period, no correlation between initial viremia level in the acute HIV infection stage and subsequent evolution of disease has been found.
Symptoms of acute HIV syndrome include:
Fever
Skin rash
Myalgia and
Pharyngitis.
These symptoms may occur less frequently in those infected via drug injection compared to those infected due to sexual contact.2
Opportunistic infections may occur during this early stage of HIV infection and may be due to both diminished CD4+ T cells and dysfunctional CD4+ T cells, secondary to viral protein and cytokine-mediated cell effects.
Immunological abnormalities are associated with acute HIV syndrome.2
These abnormalities include various anomalies in circulating lymphocyte subsets with reduction in total lymphocytes as well as CD4+ and CD8+ both noted.
Lymphadenopathy is documented in about 70% of individuals with primary acute HIV infection with most patients recovering spontaneously with many exhibiting only slightly depressed but initially stable CD4+ T cell count, prior to a progressive CD4+ T cell level decline.
Absent treatment some patients may exhibit a rapid immunologic and clinical deterioration; however, most patients exhibit clinical latency (smoldering disease).
Following acute HIV infection, several disease courses may be described (absent treatment):6
Rapid progressors (10-15%); these patients develop "late stage" symptoms in about 2-3 years.
Slow progressors (70-80%); these patients develop "late stage" symptoms in about 8-10 years.
Long-term non-progressors (5%); these patients show no decline in CD4+ T-cell counts.6
In this early phase a notable decline in circulating CD4+ T cells is observed.
An immune response to HIV-1, developing one week to three months following infection, results in a reduction of HIV-1 viral load (reduce viremia) with an increase in previously suppressed CD4+ cell number.2
For reasons discussed earlier, the immune response is insufficient to resolve the infection and persistent presence of HIV-1 in infected cells (latent) has been documented.8
![]() Clinical
latency itself may extend to 10 years, while viral replication continues
at a high level.8
Clinical
latency itself may extend to 10 years, while viral replication continues
at a high level.8
|
|
In this circumstance about 10 billion HIV virions are produced and destroyed each day.
The viral plasma half-life is about six hours with a complete virus lifecycle lasting about 2.5 days.
 |
|
A similar high turnover rate for CD4+ T lymphocytes, targeted by HIV-1, has also been described, with a half-life of an infected CD4+ lymphocyte estimated at 1.5 days.8
The uniqueness of HIV infection, compared to other human viral infections, rests in its indefinite persistence in the body, despite a cellular and humoral immune response, and its ability to emerge from a period of latency.2
(Note that herpes simplex viral infections, HSV infections, also persist but enter both a clinical and microbiological latent state).
For HIV, as noted earlier, a chronic infection is established that exhibits varying degrees of viral replication with a latent period of about 10 years prior to the untreated patient exhibiting signs of clinical disease.2
In untreated, infected patients viral replication is detectable both by direct assays for plasma HIV RNA and by identification of cell-associated HIV RNA in CD4+ T cells and macrophages both in lymphoid tissue and in the circulation.
![]() In HIV
patients undergoing treatment with cART ("effective combination
AntiRetroviral Therapy"), also known as HAART ("Highly
Active AntiRetroviral Therapy), although
plasma viremia may be suppressed to <50 HIV/ml copies, viral replication
persists.2
In HIV
patients undergoing treatment with cART ("effective combination
AntiRetroviral Therapy"), also known as HAART ("Highly
Active AntiRetroviral Therapy), although
plasma viremia may be suppressed to <50 HIV/ml copies, viral replication
persists.2
![]() HIV-Latency: CD4+
T Cell Reservoir:
HIV-Latency: CD4+
T Cell Reservoir:
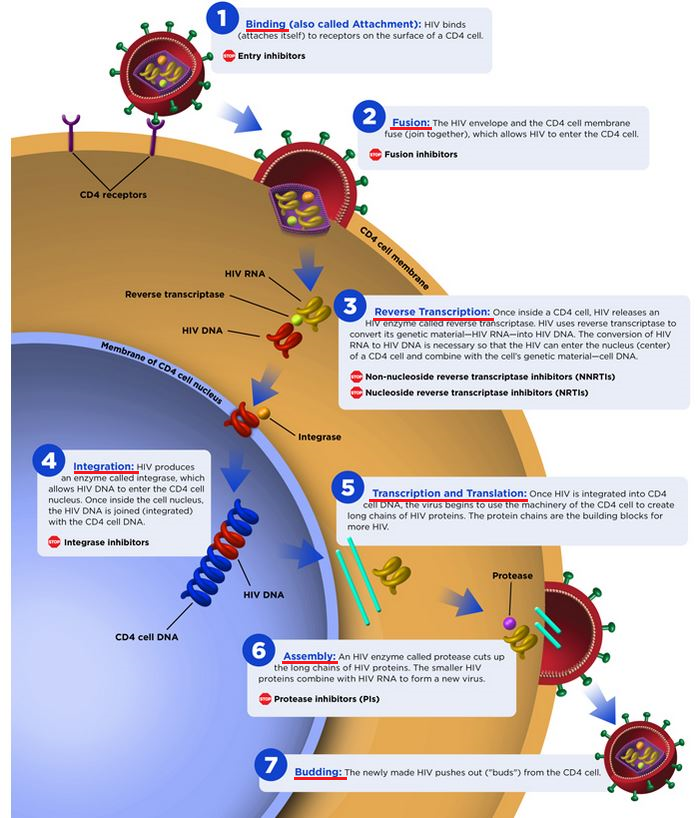
After the HIV virus has integrated into host cell DNA, postintegration latency results in some cells causing a stable, proviral reservoir.11
![]() This
reservoir is localized within resting memory CD4+ T cells.
This
reservoir is localized within resting memory CD4+ T cells.
Within a few days following the initial infection, within the acute phase, latency develops.11,13
Although the latent, integrated viral genome is not transcriptionally active, activation of the host cell allows transition of the transcriptionally inactive viral genome to a transcriptionally active viral genome fully capable of producing infectious virions.
This transition may be induced by "recall" antigens or cytokines or upon discontinuation of anti-retroviral drugs.11
Normally, na´ve CD4+ T cells remain in a resting state prior to encountering an antigen.11
After that, the CD4+ T cells become activated, proliferate and thus generate effector cells that remove the associated pathogen from the body.11
Most of these activated cells remain alive only a few weeks.
However, some of the same cells revert back to a resting state, continuing to exist as a "memory T cell" which are capable of responding at a later time to the same antigen.
The longevity of the memory T cell and their daughter cells allow these cells, if they have been infected by HIV-1, to serve as a primary reservoir for the latent HIV provirus.
![]() Accordingly,
memory T cells may be viewed as an ideal cellular HIV reservoir with the
proviral genome remaining inactive and hidden.11
Accordingly,
memory T cells may be viewed as an ideal cellular HIV reservoir with the
proviral genome remaining inactive and hidden.11
As stated above, activation of these memory T cells by reexposure to antigen or through cytokine activation (e.g. NF-κB, NFAT) , both leading to transcription factor induction, result in reactivation of latent HIV provirus.
NF-κB (Nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex controlling DNA transcription.
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |