Nursing Pharmacology: General Principles: Overview and Introduction
|
|
Drug shape
The shape of the drug is an important factor in defining the nature of the drug-receptor interaction. The three-dimensional shape of the drug is thought to interact with a complementary structural binding region of the receptor, typically a protein. The specific nature of the interaction defines whether the drug acts as an agonist promoting a change in cellular function or as an antagonist which blocks the receptor usually resulting in no direct biological effect.
For example, let's consider acetylcholine or a synthetic analogue bethanechol (Urecholine). Interaction of these molecules with receptor (nicotinic or muscarinic cholinergic receptor) causes a physiological response -- a decrease in heartbreak for instance. By contrast, a muscarinic antagonist such as atropine may bind even more tightly than acetylcholine to muscarinic receptor but causes no direct effect. However, following administration of antagonist a biological response may be observed as a result of receptor blockade.
A clinical example would be bradycardia following acute myocardial infarction. Bradycardia in this context might be due to excessive parasympathetic (cholinergic) tone and might cause unacceptably low cardiac output or predispose tomore serious arrhythmias. Administration of atropine, by blocking the muscarinic receptor blunts the action of acetylcholine and accordingly may reverse bradycardia.
Now let's consider the specific example,acetylcholine, as the 2D planar structure:
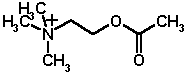
On the left side of the molecule note
the quaternary (always positively charged) Nitrogen, which is part
of the choline component of
acetylcholine. 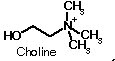 The
synthesis of acetylcholine proceeds by combination of choline and
acetate (as Acetyl-CoA)-see below
The
synthesis of acetylcholine proceeds by combination of choline and
acetate (as Acetyl-CoA)-see below
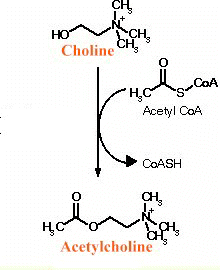
Note above the presence of an "ester" linkage [O in red] between the choline moiety and the seal group.
This ester bond is susceptible to hydrolysis, i.e. breakage which may be catalyzed by esterases (acetylcholinesterase is an example).
Acetycholine:
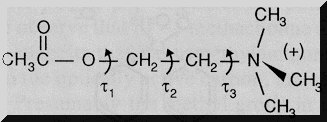
Although acetylcholine is depicted as a "static" molecule in terms of internal rotation,, acetylcholine and many other drugs exhibit free rotation around internal bonds.
For acetylcholine,tau1, tau2, tau3, represent torsion angles and refer to the degree of twist around these bonds of free rotation
Specific additional analysis is required to determine which three-dimensional form of acetylcholine appears to be preferred for binding to the cholinergic receptor. The configuration of acetylcholine and solution is quite different than the configuration when bound to the nicotinic cholinergic receptor (using two-dimensional NMR to estimate bond angles)
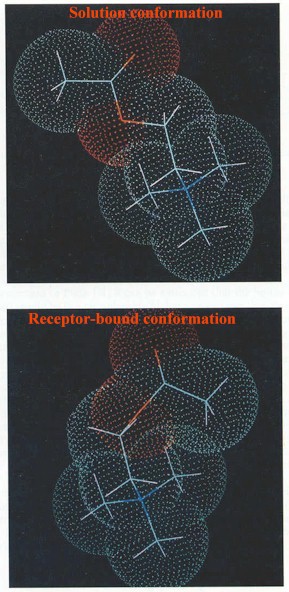
Above figures adapted from Principles of Drug Action: The Basis of Pharmacology, Third Edition, edited by William . B. Pratt and Palmer Taylor, Churchill Livingston, New York, 1990. pp 20-23. Above Acetylcholine conformation figure -- original work: Behling, RW, Yamane T, Gavon G, Jelinski LW: Conformation of Acetylcholine Bound to the Nicotinic Acetylcholine Receptor. Proc Natl Acad Sci USA 85:6721, 1988.
Some short-acting pharmacological agents are in fact short-acting because they are rapidly hydrolyzed at an ester linkage.
Ester-type local anesthetics
Esmolol (Brevibloc)
Remifentanil (Ultiva).
The biological action acetylcholine is terminated by hydrolysis, catalyzed by the enzyme acetylcholinesterase: The overall reaction is shown below --
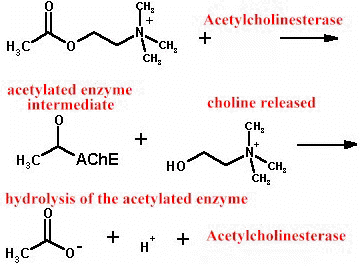
Acetylcholinesterase itself is a large, complex protein which has its primary catalytic function the extremely rapid hydrolysis of the neurotransmitter acetylcholine.
Acetylcholinesterase
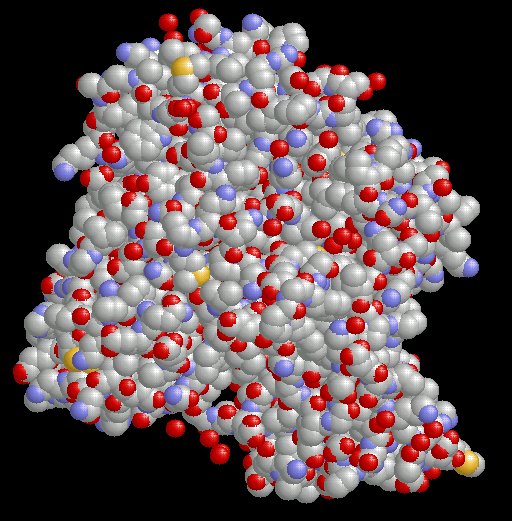
The image below illustrates the relationship between the very small molecule, acetylcholine, and its specific interaction within the very large molecule, acetylcholinesterase.
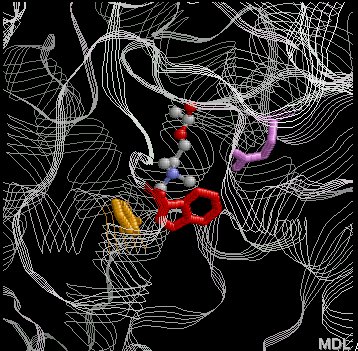
This image illustrates how the neurotransmitter acetylcholine represented above the in ball-and-stick form is recognized by specific amino acids within acetylcholinesterase's active site.
The positive charge of acetylcholine (due to the permanently positive quaternary nitrogen) interacts with tryptophan-84 (Trp-84) and phenylalanine-330 (Phe-330), through cationic (+ charged)- π-electron interactions}
This part of the acetylcholinesterase molecule is referred to as the "aromatic gorge"
The negatively charged amino acid, glutamatic acid-199 (Glu-199) is thought to interact with acetylcholine through ionic-type interactions
This image was created by Dr. Ricky Cox in the Department of Chemistry, Murray State University as part of research into the role of Noncovalent Interactions in ligand-protein interactions. Image used with permission.