|
|
|
Nursing Pharmacology Chapter 33-34: Anticancer Drugs
Natural Products
Topoisomerase Inhibitors: Topotecan (Hycamtin)
|
|
|
Topotecan (Hycamtin), a camptothecin derivative, began clinical trials in 1989.
![]() This agent was
approved as second-line treatment for:8
This agent was
approved as second-line treatment for:8
Advanced ovarian cancer (1996)
Small cell lung cancer (SCLC), following failure of initial or later chemotherapy (1998)
Stage IVB recurrent/persistent cervical cancer not suitable for surgical and/or radiotherapy + cisplatin (2006, curative intent).8
Topotecan also exhibits pharmacologic activity in management of both:7
Acute myeloid leukemia and
Myelodysplastic syndrome7
Absorption, Distribution, Metabolism, Excretion:
Aborption:7,8
![]() Topotecan (Hycamtin),
as noted with irinotecan, is administered using the intravenous (IV) route
of administration.
Topotecan (Hycamtin),
as noted with irinotecan, is administered using the intravenous (IV) route
of administration.
Topotecan is rapidly eliminated from the systemic circulation (linear pharmacokinetics) and exhibits a t1/2 of about 4 hours.1
Topotecan penetrates the blood-brain barrier allowing for CSF drug concentrations to be about 1/3 of that observed in plasma.7
Oral formulations are undergoing testing.8
The oral formulation exhibits bioavailability of about 30%-40% and is sensitive to reduced absorption across intestinal walls if converted to the active carboxylate form.
Bioavailability may also be diminished by activity of transport systems that are outward-directed.
Lastly, bioavailability may be diminished by the binding of topotecan to food, proteins or by gastrointestinal fluid-mediated drug decomposition.
In pediatric patients and young adults, topotecan volume of distribution (Vd) is about 30 L/m2 and exhibits protein binding on the order of about 35%.1,9
In pediatric patients and young adults from about six months of age to about 22 years old, significant interpatient variability (about 50%) in pharmacokinetic parameters has been reported as well as intrapatient variability on the order of 20% in these parameters.9
Topotecan is only minimally subject to hepatic metabolism.
As a consequence, patients with impaired liver function do not exhibit abnormal topotecan pharmacokinetics.
![]() By
contrast, about 1/3 of topotecan doses exhibit renal excretion.
By
contrast, about 1/3 of topotecan doses exhibit renal excretion.
Renal clearance is a significant factor in topotecan elimination requiring dosage modifications in those patients with impaired renal function.7,8
A 50% decrease in dose appears appropriate for patients exhibiting even moderate renal impairment defined as a creatinine clearance of about 20-39 mL/min).7
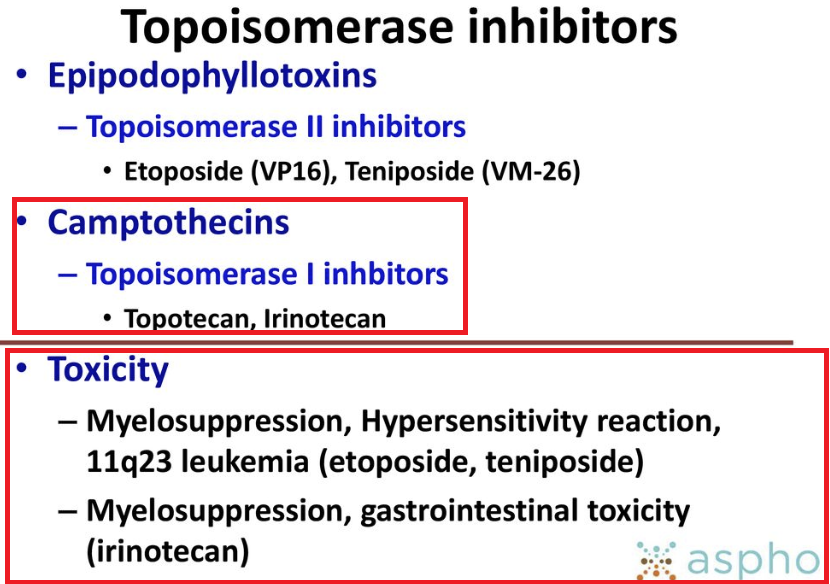
The mechanism of topotecan action follows from its effects on topoisomerase I enzyme function.1
Topoisomerase classes (I and II) act by promoting DNA strand breakage with subsequent resealing.
DNA topoisomerases modulate internal stress in supercoiled DNA such that particular regions are available for:1
Replication
Repair
Transcription.
Topotecan is a camptothecin analog that alters topoisomerase I normal function.1
The camptothecins (irinotecan and topotecan) stabilize the normally unstable transient DNA-topoisomerase I cleavable complex, thus interfering with topoisomerase I normal function.
The specific site of interference is at the religation step, leaving the initial cleavage property of topoisomerase I unaffected.
![]() A consequence
of interference with the religation step is that interaction of a DNA
replication fork with this affected DNA strand causes a permanent
double-stranded DNA break, resulting in cell death.1
A consequence
of interference with the religation step is that interaction of a DNA
replication fork with this affected DNA strand causes a permanent
double-stranded DNA break, resulting in cell death.1
Irinotecan and topotecan (campothecins) are classified as S-phase-specific agents since active DNA synthesis is required for drug-induced cytotoxicity.
![]() A clinical
consequence is that these cytotoxic agents must be exposed to tumor cells
for an extended period of time, ensuring that the camptothecin is present
when a given tumor cell is dividing and present at an appropriate
therapeutic dose level.1
A clinical
consequence is that these cytotoxic agents must be exposed to tumor cells
for an extended period of time, ensuring that the camptothecin is present
when a given tumor cell is dividing and present at an appropriate
therapeutic dose level.1
The most common dose-limiting toxicity associated with topotecan is myelosuppression.1
Topotecan -induced myelosuppression is more likely to occur if the patient has received significant previous radiotherapy or other anticancer agents which suppress bone marrow.
Neutropenia due to topotecan administration may present in the presence or absence of thrombocytopenia.1
The incidence of neutropenia may reach 80% (dosing schedule: 1.5 mg/m2 every day for 5 days every 3 weeks).
Febrile neutropenia may be associated with an incidence at about 25%.
For patients experiencing hematological cancers, G.I. side effects including mucositis and diarrhea may well be dose-limiting.1
Other topotecan-related adverse effects include:
Nausea and vomiting
Fever
Fatigue
Alopecia7
Rash
Increased liver transaminase levels.1
 |
|
One mechanism involved in cellular resistance to anticancer effects for topoisomerase inhibitors is based on mechanisms that decrease cellular drug accumulation.1
For example, topotecan is a substrate for the P-glycoprotein multidrug transporter (P-glycoprotein (ABCB1) which supports efflux of a variety of hydrophobic agents including drugs.
By contrast, irinotecan is not a substrate for this transport system.
As a class, camptothecin resistance could occur also from reduced expression or an abnormality (mutation) in its target, topoisomerase I.1
|
|
Cervical cancer: topotecan (Hycamtin) has been FDA approved for treating stage IVB persistent, recurrent cervical carcinoma in patients in which curative surgical and/or radiotherapy with curative intent is not possible.7
Ovarian cancer
Small-cell lung cancer
Acute myeloid leukemia: topotecan may be used in treating AML (with a Class 2B designation).
Myelodysplastic syndrome: topotecan may be used in MDS (with the Class 2B designation).7
Phase II clinical trials have demonstrated topotecan antitumor activity when given as a single agent with various administration protocols in: 8
Ovarian cancer
Small-cell lung cancer
Non-small cell lung cancer
Breast cancer
Myelodysplastic syndrome
Chronic myelomonocytic leukemia.8
Some, limited anti-cancer activity has been noted in 8
Head and neck cancer
Pancreatic cancer
Gastric cancer
Esophageal cancer
Prostate cancer
Hepatocellular carcinoma
Recurring
malignant glioma.8