|
|
|
Chapter 7: Antihypertensive Drugs
Overview:
About 70 million Americans exhibit blood pressures above normal, which corresponds to a systolic blood pressure of = >140 mmHg Or a diastolic pressure of =>90 mmHg. Two thirds of these individuals are aware of their diagnosis; however, somewhat less than half (45%) receive treatment and only about a third receive treatment sufficient to lower their blood pressure to at least the reference norm of 140/90 mmHg.2
The likelihood of hypertension increases with age and appears more likely to occur in blacks compared to whites.
Two of the major complications of hypertension, stroke and coronary vascular disease, can be lethal; however, these complications have, over the past 30 years, exhibited reduced frequency (about 50% less common).2
Unfortunately, this decline in frequency appears to have leveled off; however, heart failure and end-stage renal disease, also complications of hypertension, have continued to increase in liklihood.2
Although the common focus on systolic and diastolic blood pressure remains important, for those individuals over 50 years old cardiovascular complications appear better predicted by systolic pressure and pulse pressure compared to diastolic pressure.2
Generally, a single reading above the 140/90 mmHg threshold is not considered sufficient for a definitive diagnosis of hypertension.
However, there are exceptions to this rule. For example, sometimes patients present with hypertension with clear evidence of life-threatening, end-organ damage. In other cases, a blood pressure reading of >220/125 mmHg defines hypertensive urgency even if life-threatening end-organ damage is not apparent.
Most of the time, however, hypertension is a diagnosis defined by repetitive blood pressure measurements, given that readings can be variable and patients exhibiting higher readings are the individuals most likely to exhibit blood pressure readings transitioning towards normal upon repetitive examinations.
Depending on the initial blood pressure value, excessive delay in establishing a diagnosis does carry some risk. In high-risk individuals even a three-month delay in initiating treatment may correspond to a 200% increase in cardiovascular morbidity/mortality.2
| Diuretics | Sympatholytics | Vasodilators | Calcium Channel Blockers | Angiotensin Converting Enzyme (ACE) Inhibitor |
![]()
Thiazides
|
Potassium Sparing
|
Loop Diuretics
|
Diuretics: Introduction
As noted above, there are three principal types of diuretics: the thiazide agents, examples of which include chlorthalidone and the prototypical agent chlorothiazide/hydrochlorothiazide; the loop diuretics such as furosemide, bumetanide, torsemide and ethacrynic acid. Finally, the K+-sparing agents such as amiloride, triamterene, and spirolactone.
At least as a premise, the use of diureticsrepresents an extension of the idea of hypertension management by limitation of sodium ingested in diet. The thiazides as a group are effective diuretics and also improve the action of other antihypertensive drugs. Clinical trials have demonstrated that diuretics continue to be central for management of the hypertensive patient.1
Although the initial hypotensive effects of thiazide diuretics may be explained by the reduced extracellular volume resulting in reduced cardiac output. Hypotensive effects, however, remains one following normalization of cardiac output. The mechanism is most likely a reduction in systemic vascular resistance.1
Chlorothiazide (Diuril)
The thiazides act in the distal tubule to decrease sodium reabsorption (inhibits Na/Cl transporter).
As a result of decreased sodium and chloride reabsorption, a hyperosmolar diuresis ensues.
Delivery of more sodium to the distal tubule results in potassium loss by an exchange mechanism.
Thiazides also promote calcium reabsorption, in contrast to loop diuretics.
The initial decrease in blood volume followed by a longer-termed reduction in vascular resistance appear to account for the hypotensive effects of the thiazides.
Adverse Effects
Potassium depletion is a potentially serious side-effect that may require potassium supplementation and/or concurrent use of potassium-sparing diuretics.
Hyperuricemia may occur precipitating gout.
The increase in systemic uric acid is due to a decrease in the effectiveness of the organic acid secretory system.
Diabetic patient may have difficulty in maintaining proper blood sugar levels.
|
|
|
Furosemide (Lasix), Bumetanide (Bumex),Ethacrynic acid (Edecrin)
Furosemide (Lasix), bumetanide (Bumex), and ethacrynic acid (Edecrin) are "high-ceiling" loop diuretics acting primarily at the ascending limb of the loop of Henle.
The effectiveness of these agents is related to their site of action because reabsorption of about 30 - 40% of the filtered sodium and chloride load occurs at the ascending loop.
Distal sites are not able to compensate completely for this magnitude of reduction of NaCl reabsorption.
Loop diuretics increase urinary Ca2+ in contrast to the action of thiazides.
Loop diuretics also increase renal blood flow by decreasing renal vascular resistance.
These drugs are rarely used in the management of hypertension because of their short duration of action and the availability of better drugs.
![]() Adverse Effects
Adverse Effects
Ototoxicity
Furosemide (Lasix) and ethacrynic acid (Edecrin) block renal excretion of uric acid by competition with renal secretory and biliary secretory systems.
Therefore, these agents can precipitate gout.
Potassium depletion.
|
|
|
![]()
Centrally Active
|
Adrenergic Neuron Blocker |
Adrenoceptor Antagonists |
Clonidine (Catapres) (Sympatholytic)
Antihypertensive:
Clonidine (Catapres) acts in the brain, inhibiting adrenergic outflow from the brainstem. Inhibition of sympathetic outflow results in a decrease in blood pressure.
Mechanism of action: centrally acting selective a2 adrenergic agonist.
Especially effective in
management of severe hypertension or
in renin-dependent hypertension
Transdermal clonidine (Catapres) patch: useful for surgical patients unable to take oral formulation
Clonidine (Catapres) reduces cardiac output (by reducing both stroke volume and heart rate) and peripheral resistance.
Reduction in stoke volume occurs due to increased venous pooling (decreased preload).
Clonidine (Catapres) does not interfere with cardiovascular responses to exercise.
Renal blood flow and function is maintained during clonidine treatment.
Clonidine (Catapres) has minimal or no effect on plasma lipids.
Other Clinical Uses
Analgesia--
Preservative-free clonidine administered into epidural/subarachnoid space (150-450 micrograms)-- dose-dependent analgesia
No respiratory depression, nausea, vomiting, delayed gastric emptying or pruritus -- effects associated with opioids
Probable Mechanism: activation postsynaptic a2 receptors in the spinal cord substantia gelatinosa
Clonidine (Catapres) & morphine: no cross-tolerance when used concurrently in neuraxial analgesia
Side effects of neuraxial clonidine (Catapres)
hypotension, sedation, dry mouth
Preanesthetic Medication:
Oral clonidine (Catapres) (preanesthetic medication):
Enhances intrathecal morphine + tetracaine (pontocaine) for postoperative analgesia (no increase in morphine-related side effects)
Preanesthetic clonidine (Catapres) also:
Reduces reflex tachycardia that may be caused by direct laryngoscopy for tracheal intubation
Reduces intraoperative blood-pressure heart rate lability
Reduces plasma catecholamines levels
Significantly decreases anesthetic requirements for inhaled (MAC) and injected agents.
![]() Adverse Effects
Adverse Effects
Dry Mouth (xerostomia)
bradycardia (in patients with SA nodal abnormality)
Withdrawal syndrome upon abrupt discontinuation (increased blood pressure, headache, tachycardia, apprehension, tremors
|
|
|
|
|
|
Stoelting, R.K., "Antihypertensive Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 302-312.
![]()
Methyldopa (Aldomet) (Sympatholytic)
Methyldopa (Aldomet) is a prodrug which is metabolized to the active agent, alpha-methylnorepinephrine.
Alpha-methylnorepinephrine acts in the brain, inhibiting adrenergic outflow from the brainstem. Inhibition of sympathetic outflow results in a decrease in blood pressure.
Methyldopa (Aldomet) produces no change in cardiac output in younger patients, but in older patients a decline in cardiac output results from reduced heart rate and stroke volume.
The reduction in stroke volume occurs due to increased venous pooling (decreased preload).
Since renal blood flow and function is maintained during methyldopa treatment, methyldopa maybe valuable in managing hypertensive patients with renal insufficiency.
![]() Adverse Effects
Adverse Effects
Dry Mouth
Parkinsonian signs
Reduced libido
Hyperprolactinemia (gynecomastia, galactorrhea)
Bradycardia (in patients with SA nodal abnormality)
Hepatoxicity (avoid in patients with hepatic disease)
Positive Coombs' test (20%)
1-5% of those with positive Coombs' develop hemolytic anemia (requiring immediate discontinuation of the drug)
|
|
|
![]()
Guanethidine (Ismelin) & Guanadrel (Hylorel)
Guanethidine (Ismelin) inhibits the function of postganglionic adrenergic neurons, thus inhibiting sympathetic function.
Guanethidine (Ismelin) uses the norepinephrine (N.E.) re-uptake transporter to reach its site of action, the neurosecretory vesicles.
Guanethidine (Ismelin) replaces norepinphrine in the vesicle and is released instead of the normal transmitter.
Guanethidine (Ismelin) is an inactive transmitter and the replacement of N.E. by an inactive agent is responsible for its antihypertensive effects (maintenance dosing).
Adrenergic blockade by guanethidine (Ismelin) results in post-synaptic supersensitivity.
Sympathetic blockade by guanethidine (Ismelin) produces:
venodilatation
reduction in cardiac output due to inhibition of cardiac sympathetic innervation
blockade of the sympathetic reflex arteriolar response to the reduction in cardiac output.
![]() Adverse Effects
Adverse Effects
Symptomatic hypotension (due to sympathetic reflex blockade)
Sexual dysfunction (delayed ejaculation)
Diarrhea
Guanethidine (Ismelin) effects blocked by N.E. reuptake blockers (tricyclic antidepressants, cocaine, ephedrine, amphetamine, chlorpromazine (Thorazine))
![]()
Reserpine (Adrenergic Neuron Blocker)
Reserpine inhibits the function of postganglionic adrenergic neurons, thus inhibiting sympathetic function.
Reserpine binds to noradrenergic storage vesicles in central and peripheral sympathetic nerve terminals.
Storage vesicles become nonfunctional as a result of interacting with reserpine and lose the ability to store and concentrate norepinephrine (N.E.) and dopamine.
N.E. and dopamine leaking from vesicles are enzymatically destroyed in the cytoplasm and as a consequence little transmitter is released upon nerve ending depolarization.
Depletion of transmitter in both the central and peripheral nervous system suggest that both sites by be important mediators of the antihypertensive response.
Chronic adrenergic neuronal blockade by reserpine results in a reduction of cardiac output and peripheral vascular resistance.
![]() Adverse Effect
Adverse Effect
CNS effects predominate, including sedation, inability to concentrate, and depression.
![]()
Labetalol (Trandate, Normodyne)
Labetalol (Trandate, Normodyne) is a competitive antagonist at both a1 and ß1-adrenergic receptors. It also has an intrinsic sympathomimetic effect at ß2 receptors
Antihypertensive effects of labetalol results from actions at both alpha1 and beta-adrenergic receptors.
alpha1 receptor blockade results in vasodilatation which is further enhanced by ß2 receptor activation.
A reduction in heart rate is mediated by beta1 receptor antagonism.
Labetalol (Trandate, Normodyne) does not alter serum lipids.
![]() Adverse Effects
Adverse Effects
Orthostatic hypotension may occur due to alpha1 receptor blockade.
Liver injury has been reported with labetalol (Trandate, Normodyne) usage.
Labetalol (Trandate, Normodyne) metabolites: false positive for pheochromocytoma
Paresthesias (scalp tingling)
Bronchospasm -- incidence similar to that observed with metoprolol (Lopressor) or atenolol (Tenormin)
urinary retention
|
|
|
Stoelting, R.K., "Antihypertensive Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 302-312.
![]()
Prazosin (Minipress) (alpha1-Arenoceptor Antagonis); Terazosin (Hytrin) (alpha1-Adrenoceptor Antagonist)
Prazosin (Minipress), terazosin (Hytrin), and doxazosin (Cardura) reduce arteriolar resistance and increase venous capacitance as a consequence of alpha1 adrenergic receptor blockade.
Normal inhibition of norepinephrine-mediate inhibition through alpha2 receptors remain-- prazosin (Minipress) is a selective postsynaptic alpha1 adrenergic receptor blocker
The short-term increase in heart rate and plasma renin levels do not persist although the vasodilation continues.
Prazosin (Minipress) monotherapy --less effective than thiazide diuretics
Prazosin (Minipress) in combination with other agents: quite effective in young patients with moderately severe hypertension
Good patient compliance
Cardiovascular Effects: --
Prazosin (Minipress) reduces systemic vascular resistance without:
causing reflex-mediated tachycardia
causing increases in plasma renin {as seen with minoxidil/hydralazine}
absence of changes in plasma renin reflect continued alpha2 receptor function which normally inhibits renin release {recall that prazosin is an alpha1 selective antagonist}
Prazosin (Minipress) -- greater affinity for venular alpha receptors compared to arteriolar alpha receptors; resultant hemodynamics effect (orthostatic hypotension) --an action more similar to nitroglycerin than hydralazine (Apresoline).
Renal blood flow is maintained.
Retention of salt and water occurs.
Alpha1-adrenergic receptor blockers reduce plasma triglycerides, total and LDL-cholesterol, and increase HDL-cholesterol.
Other Therapeutic Uses:
Congestive heart failure: valuable for reducing afterload
Preoperative preparation of patients with pheochromocytoma
Treatment of benign prostatic hypertrophy in older males (drug decreases prostate size)
![]() Adverse Effect
Adverse Effect
Inital-dose marked orthostatic hypotension is seen in about 50% of cases-- (sudden syncope; dosage dependence)
Fluid retention, vertigo
dry mouth, urinary frequency, lethargy, sexual dysfunction, nasal congestion, nightmares
Anesthetic Implications:
Prazosin (Minipress)-induced alpha1 blockade may cause exaggerated hypotension during epidural anesthesia (alpha receptor--blockade prevents compensatory vasoconstriction)
Prazosin (Minipress)-exacerbated hypotension may not be responsive to typical alpha1 adrenergic agonists (e.g. phenylephrine) dosage; epinephrine may be required to increase systemic vascular resistance & BP in this setting
The combination of prazosin (Minipress) and a beta-blocker could result in nearly refractory hypotension during regional anesthesia (diminished response to both beta and alpha1 agonists)
Stoelting, R.K., "Antihypertensive Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 302-312.
![]()
| Diazoxide (Hyperstat) | Hydralazine (Apresoline) | Minoxidil (Loniten) | Nitroprusside sodium (Nipride) |
![]()
Diazoxide (Hyperstat), Nitroprusside sodium (Nipride)
![]() Vasodilators used for acute
management of hypertensive crisis or malignant
hypertension include sodium nitroprusside and diazoxide.
Vasodilators used for acute
management of hypertensive crisis or malignant
hypertension include sodium nitroprusside and diazoxide.
Nitroprusside sodium (Nipride) is the agent of choice.
Administered by a continuously variable rate i.v. infusion pump, precise blood pressure control can be obtained.
Nitroprusside sodium (Nipride), a nitrovasodilator, is metabolized by smooth muscle cells to nitric oxide which dilates both arterioles and venules.
Side effects are mainly due to excessive vasodilation.
Much less commonly, toxicity may result from conversion of nitroprusside to cyanide and thiocyanate.
Risk of toxicity due to thiocyanate increases after 24 to 48 hours.
Nitroprusside sodium (Nipride) can worsen arterial hypoxemia in patients with obstructive pulmonary airway disease since nitroprusside will interfere with hypoxic pulmonary vasoconstriction. A result is increasing ventilation-perfusion mismatching.
Diazoxide (Hyperstat) is infrequently used unless accurate infusion pumps are unavailable.
The mechanism of action involves activation of ATP-sensitive potassium channels, hyperpolarization of arteriolar smooth muscle, relaxation and dilation.
Adverse effects include salt and water retention and hyperglycemia. Diazoxide inhibits insulin release.
|
|
|
![]()
Hydralazine (Apresoline) Minoxidil (Loniten)
Vasodilators used for chronic treatment include hydralazine (Apresoline) and minoxidil (Loniten).
These drugs are not typically administered as monotherapy due to significant reflex-mediated cardiac stimulation and water retention. Instead they may be combined with sympatholytic drugs.
Adverse effects include those induced by vasodilation such as:
hypotension
palpitation
tachycardia
fluid retention
headache
angina
A drug-induced lupus syndrome is associated with hydralazine (Apresoline).
A drug-induced hypertrichosis is associated with minoxidil (Loniten).
![]()
|
|
Amlodipine (Norvasc) Felodipine (Plendil)
Calcium channel blockers are effective in treating hypertension because they reduce peripheral resistance.
Amlodipine (Norvasc) and Felodipine (Plendil) have relatively little effects on reducing myocardial contractility compared to verapamil (Isoptin, Calan) or diltiazem (Cardiazem).
Arteriolar vascular tone depends on free intracellular Ca2+ concentration.
Calcium channel blockers reduce transmembrane movement of Ca2+
reduce the amount reaching intracellular sites
and therefore reduce vascular smooth muscle tone.
All calcium channel blocks appear similarly effective for management of mild to moderate hypertension.
For low-renin hypertensive patients (elderly and African-American groups), Ca2+ channel blockers appear good choices for monotherapy (single drug) control.
Adverse Effects
SA nodal inhibition may lead to bradycardia or SA nodal arrest.
This effect is more prominent if beta adrenergic antagonists are concurrently administered.
GI reflux.
Negative inotropic are augmented if beta-adrenergic receptor antagonists are concurrently administered.
Calcium channel blockers should not be administered if the patient has SA or AV nodal abnormalities or in patients with significant congestive heart failure.
|
|
|
![]()
Overview
Highly lipid-soluble nefedipine analog
Ready access to the CNS -- reduces large cerebral arterial contraction
Clinical Use:
Cerebral Vasospasm:
Useful in preventing/reducing cerebral vasospasm associated with subarachnoid hemorrhage
Vasospasm -- mediated by calcium ion influx
Nimodipine (Nimotop) administered over a three week course (oral administration) results and decreased frequency of neurologic defects secondary to cerebral vasospasm in subarachnoid hemorrhage patients.
For comatose patients: deliver through nasogastric tube
Side effects/Concerns:
systemic hypotension (with excess nimodipine (Nimotop) effect)
possible increase in intracranial pressure -- especially in patients with decreased intracranial compliance
Stoelting, R.K., "Calcium Channel Blockers", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, p. 350.
![]()
Diltiazem (Calcium Channel Blocker)
Calcium channel blockers are effective in treating hypertension because they reduce peripheral resistance.
Arteriolar vascular tone depends on free intracellular Ca2+ concentration.
Calcium channel blockers reduce transmembrane movement of Ca2+ , reduce the amount reaching intracellular sites and therefore reduce vascular smooth muscle tone.
All calcium channel blocks appear similarly effective for management of mild to moderate hypertension
.For low-renin hypertensive patients (elderly and African-American groups), Ca2+ channel blockers appear good choices for monotherapy (single drug) control.
Diltiazem has a direct negative chronotropic effect on the heart sufficient to block reflex-mediated tachycardia secondary to the decrease in peripheral resistance.
The reflex-mediated adrenergic stimulation tends to counteract negative inotropic properties of diltiazem.
Adverse Effects
SA nodal inhibition may lead to bradycardia or SA nodal arrest.
This effect is more prominent if beta adrenergic antagonists are concurrently administered.
GI reflux.
Negative inotropic are augmented if beta-adrenergic receptor antagonists are concurrently administered.
Calcium channel blockers should not be administered if the patient has SA or AV nodal abnormalities or in patients with significant congestive heart failure.
|
|
|
![]()
 |
![]()
|
***angiotensin receptor blocker |
Captopril and other Angiotensin Converting Enzyme Inhibitors
Pharmacological Properties
Angiotensin II, a potent vasoconstrictor, is produced by the action of angiotensin converting enzyme (ACE) on the substrate angiotensin I. Angiotensin II activity produces
a rapid pressor response
a slow pressor response and
vascular and cardiac hypertrophy and remodeling.
Antihypertensive effects of ACE inhibitors are due to the reduction in the amount of angiotensin II produced.
ACE inhibitors are efficacious in management of hypertension and have a favorable side effect profile.
ACE inhibitor are advantageous in management of diabetic patients by reducing the development of diabetic neuropathy and glomerulosclerosis.
ACE inhibitor are probably the antihypertensive drug of choice in treatment of hypertensive patient who have hypertrophic left ventricles.
Hypertensive patients who have ischemic heart disease with impaired left ventricular function also benefit from ACE inhibitor treatment.
ACE inhibitors reduce the normal aldosterone response to sodium loss (normally aldosterone opposes diuretic-induced sodium loss).
Therefore, the use of ACE inhibitors enhance the efficacy of diuretic treatment, allowing the use of lower diuretic dosages and improving control of hypertension.
If diuretics are administered at higher dosages in combination with ACE inhibitors significant and undesirable hypotensive reactions can occur with attendant excessive sodium loss.
Reduction in aldosterone production by ACE inhibitors also affects potassium levels.
The tendency is for potassium retention, which may be serious in patients with renal disease or if the patient is also taking potassium sparing diuretics, nonsteroidal anti-inflammatory agents or potassium supplements.
Captopril (Capoten): not a prodrug
Enalapril (Vasotec): prodrug
Lisinopril (Prinvivil, Zestril): not a prodrug
Ramipril (Altace): prodrug
Captopril (Capoten)
Overview
Orally effective, competitive inhibitor of angiotensin I-converting enzyme (peptidyl dipeptidase) [enzyme converts angiotensin I to angiotensin II (active)]
Decreases circulating angiotensin II & aldosterone {angiotensin II stimulates aldosterone secretion by the adrenal cortex}
Compensatory response: increase in angiotensin I & increased renin levels {loss of negative feedback control}
Decrease in aldosterone cause slight increase in serum potassium
Pharmacokinetics:captopril
well absorbed; 25%-30% protein bound
rapid converting enzyme inhibition (within 15 minutes following oral administration)
50% drug excreted unchanged
elimination half-life: two hours -- oxidation, real excretion
Cardiovascular Effects: captopril (Capoten)
Decrease systemic vascular resistance (secondary to a decrease in Na+ & water retention)
Prominent decrease in renal vascular resistance
Reduced systemic blood pressure: no change in heart rate & cardiac output
Absence of heart rate change despite reduced blood pressure may suggest alteration baroreceptor sensitivity
No orthostatic hypotension (captopril does not interfere with sympathetic nervous system function)
captopril may improve vasodilator drug treatment efficacy in management of CHF by blocking vasodilator-induced increases in renin secretion
Adverse Effects
Angioedema, although rare, may be potentially fatal
ACE inhibitiors should not be used during pregnancy
Dry cough
In renovascular hypertension, glomerular filtration pressures are maintained by vasoconstriction of the post-glomerular arterioles, an effect mediated by angiotensin II. Used of ACE inhibitors in patients with renovascular hypertension due to bilateral renal artery stenosis can therefore precipitate a significant reduction in GFR and acute renal failure.
Initial dose of an ACE inhibitor may precipitate an excessive hypotensive response.
Angiotensin II receptor antagonists: Losartin (Cozaar) and Irbesartin, AT1 angiotensin II receptor antagonists, act by blocking the interaction between angiotensin II and its receptor.
The magnitude of the blood pressure decrease associated with losartin may be somewhat less than that seen with ACE inhibitors.
![]()
Essential Hypertension (greater than 90% of cases)
Occurs in patients who have arterial hypertension without a clear, definable cause.
Essential hypertension is also referred to as primary or idiopathic hypertension.
Classification of Arterial Hypertension
I. Systolic hypertension (with wide pulse pressure) can be caused either by conditions of decreased aortic compliance, often due to arteriosclerosis or to increased stroke volume.
Some conditions that increase stroke volume:
Thyrotoxicosis
Fever
Aortic regurgitation
II. Systolic and Diastolic hypertension (increased peripheral vascular resistance)
Renal
Renovascular stenosis or renal infarction
Polycystic kidney disease
Acute and chronic glomerulonephritis
Chronic pyelonephritis
Renin-producing tumors
Other severe renal diseases)
Endocrine
Adrenocortical hyperactivity (Cushing's disease, primary hyperaldosteronism)
Oral contraceptives
Pheochromcytoma
Acromegaly
Myxedema
Neurogenic
Psychogenic
Polyneuritis
Elevated intracranial pressure
Familial dysautonomia
Miscellaneous
Increased intravascular volume
Polyarteritis nodosa
Hypercalcemia
Aortic coarctation
Hypertension of Unknown Etiology
Toxemia of pregnancy
Acute intermittant porphyria
Essential Hypertension (> 90% of all cases of hypertension
![]()
Arterial pressure is determined by a number of interacting factors
Physiological Factors Influencing Arterial Pressure
Preload & Contractility:
As blood volume returning to the heart increases, preload increases and there is enhanced filling with ventricular dilation.
According to Starling's Law, increased ventricular stretch usually leads to increased contractility.
Increased preload and increased contractility lead to increased stroke volume and ultimately an increase in arterial pressure, all other factors remaining equal.
Some antihypertensive drugs decrease preload.
Heart rate: Since the product of heart rate and stroke volume equals cardiac output, an increase in heart rate will increase arterial blood pressure, all other factors remaining equal.
Some antihypertensive agents decrease heart rate (ß-adrenergic receptor antagonists, e.g.).
Heart Rate X Stroke Volume = Cardiac Output
Cardiac Output X Peripheral Resistance = Arterial Pressure
Peripheral resistance: For a given cardiac output, blood pressure depends only on peripheral resistance.
Some antihypertensive drugs act to reduce peripheral resistance.

Depending on mechanism of action, a given antihypertensive may:
Reduce preload
Reduce afterload
Decrease heart rate
Reduce peripheral resistance
Reduce contractility.
Many antihypertensive drugs have multiple effects.
![]()
Antihypertensive Drugs and Anesthesia
There is probably excessive concern about interaction potential between anesthetics and antihypertensive drugs.
Areas of concern for administration of anesthetic to patients treated with antihypertensive medications:
Reduced sympathetic nervous system activity--manifestations:
Orthostatic hypotension
Excessive systemic blood-pressure responses (decreases) to:
acute blood loss
body position changes
decreased venous return cause by positive-pressure ventilation
Reduced sensitivity to indirect-acting sympathomimetic agents (caused by antihypertensive drugs that deplete norepinephrine from nerve terminals
Possible enhanced response to catecholamines and direct-acting sympathomimetics following sympathetic nervous system blockade (reduced alpha-adrenergic receptor tone with loss of tonic stimulation)
Altered physiological response to sympathomimetic agents
Sedation
Maintenance of antihypertensive drug treatment during perioperative time frame:
Fewer systemic blood-pressure and heart rate fluctuations during anesthesia
Decreased likelihood of cardiac dysrhythmias
Conclusion:
Previously effective antihypertensive drug therapy should be continued during the perioperative period.
The pharmacology of the particular antihypertensive drug should be considered in the development of the anesthesia plan.
Stoelting, R.K., "Antihypertensive Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 302-312.
Considerations: Anesthetic Management in Hypertensive Patients
Hypertension: Organ Systems Effects
Normal control of BP: sympathoadrenal axis-- response to a decrease in BP
Sensed by Central baroreceptors {heart & great arteries}
Stimulation of ß-adrenergic systems
increased heart rate (positive chronotropic response)
increased force of contraction (contractility, positive inotropic response)
increased renin secretion {juxtaglomerular renal cells}
Stimulation of alpha-adrenoceptor systems: causes vasoconstriction
With essential hypertension, above mechanisms function inappropriately
Excessive sympathetic activation
Elevated norepinephrine may promote through vascular endothelium injury:
vascular hypertrophy
atherogenesis
ß-adrenergic receptor down-regulation
Reduced endothelium-mediated vascular relaxation
Consequence: increased vasoconstrictive tone (chronic vasoconstriction)
Excessive sympathetic activation promotes enhanced peripheral vascular resistance in hypertensive patients
Hypertension and the kidney:
ß-adrenergic receptor stimulation leads to increased renin secretion which in turn leads to increased angiotensin II levels
Increased angiotensin II levels promotes an increase in BP by means of:
direct vasoconstriction
increasing renal cortical {zona glomerulosa}aldosterone production which causes intravascular volume expansion
Hypertension associated with elevated renin levels may predispose patients to myocardial infarction {compared to those patients with normal/decreased renin levels}
Hypertension associated with elevated renin levels is amenable to treatment with ACE inhibitors {e.g., captopril (Capoten)}
"Pressure-naturesis"-dysfunctional in hypertensive kidneys {Definition of pressure-naturesis: increasing Na+ & H2O excretion by the kidney with increased blood-pressure}
Frequency: 4%-5% of hypertensive patients
Principal causes:
Endocrine
Renal
Endocrine causes of secondary hypertension
Cushing's Syndrome--Cortisol overproduction
Adrenal hyperplasia:
Secondary to excessive pituitary ACTH production
Pituitary-hypothalamic dysfunction
Pituitary ACTH-producing micro-or macroadenomas
Secondary to ACTH or CRH producing nonendocrine tumors
Bronchogenic carcinoma
Thymic carcinoma
Pancreatic carcinoma
Bronchial adenoma
Adrenal nodular hyperplasia
Adrenal neoplasia
Adenoma
Carcinoma
Exogenous, iatrogenic causes (most common cause)
Long-term glucocorticoid use
Long-term ACTH use
bilateral adrenal hyperplasia due to pituitary adenoma (basophilic tumor)
about 20 to 25 percent of Cushing's syndrome patients have adrenal neoplasm
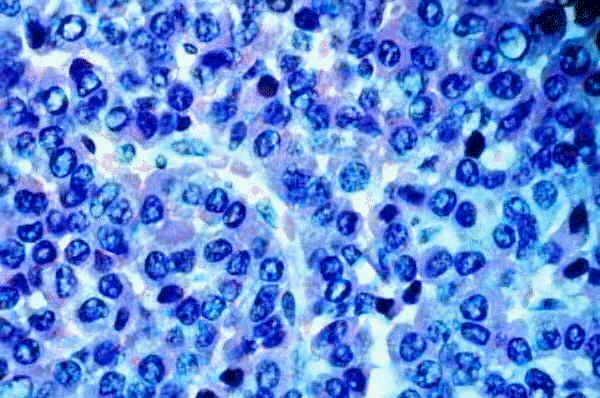
"Adenoma composed of basophilic cells (blue with H&E stain) in Cushing disease."
(c) 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center.
Cushing's disease-Presenting Symptoms: Hypertension, Truncal obesity, Glucose intolerance, Muscle weakness, Striae*
*"Stretch marks on the skin with a silvery-white hue. Often the result of stretching the skin in pregnancy. Heavy or long-term
corticosteroid use (cream or oral forms) can cause striae formation.-on-line medical dictionary"
Hyperaldosteronism
Twice as common in women as in man
Most often presents between 30 in 50 years of age
Adrenal adenoma -- excessive aldosterone production
unilateral adenoma (usually small; either side)
Conn's syndrome (primary hyperaldosteronism;Two types of abnormalities are noted: (1) benign tumor of one adrenal (adenoma) or (2) hyperplasia., a general enlargement of both adrenals.
hyperplastic adrenal glands -- abnormal secretion
malignant tumor
adrenal carcinoma (rare)
Physiological Effects of aldosterone hypersecretion:
increased renal distal tubular exchange of sodium for secreted potassium and hydrogen ions -- body potassium depletion/hypokalemia
Diagnosis--Criteria:
diastolic hypertension (no edema)
renin hyposecretion (low plasma renin activity)
renin secretion does not increase with volume depletion
aldosterone hypersecretion that is not suppressed with volume expansion
Diastolic hypertension (not very severe)
secondary to increased sodium reabsorption/volume expansion
Headaches
Polyuria, polydipsia
impairment of urinary concentrating ability
Weakness
due to effects of potassium depletion
Tetany
Electrocardiographic changes -- consistent with potassium depletion (hypokalemia-- which increases ectopy)
prominent U waves
cardiac arrhythmias
premature contractions
Many effects secondary to potassium loss associated with:
hypokalemia
may be severe (< 3 mmol/L)
hypernatremia-- due to:
sodium retention
water loss from polyuria
metabolic alkalosis-- due to
urinary hydrogen ion loss
movement of hydrogen ion into potassium-depleted cells
alkalosis enhanced by potassium deficiency which increases proximal convoluted tubule capacity to reabsorb filtered bicarbonate.
Cause: inappropriate increased aldosterone production due to renin-angiotensin system activation
Accelerated hypertension
Edema
normal physiologic response to estrogen-induced increased plasma renin substrate and plasma renin activity and to antialdosterone actions of progestogens
in hypertension -- cause:
overproduction of renin (primary reninism)
renin overproduction secondary to reduced renal blood flow/perfusion pressure
reduced renal artery lumen secondary to atherosclerosis or fiber muscular hyperplasia
severe arteriolar nephrosclerosis (malignant hypertension)
profound renal vasoconstriction (accelerated hypertension)
may be caused by rare renin-producing tumors (primary reninism)
juxtaglomerular cell tumor
Physiological characteristics:
increased plasma renin activity
moderate/marked increases in aldosterone levels
hypokalemic alkalosis
Pheochromocytoma
Pheochromocytoma
Occurs as:
a singular tumor or as
an element of multiple endocrine neoplasia {including thyroidal medullary carcinoma and hyperparathyroidism}
Associated with elevated catecholamine levels
Surgical manipulation {during tumor removal} is likely to cause a rapid increase in blood-pressure due to releases of catecholamines.
Patients with pheochromocytoma may have reduced blood volume-a factor to consider in perioperative management
Undiagnosed pheochromocytoma in patients presenting intraoperatively with hypertension is associated with high mortality {50%}
Pharmacological management prior to tumor removal
alpha & ß-adrenoceptor blockers (a blockers to reduce vasoconstriction; ß-blockers decrease heart and contractility, also reducing blood pressure) -- both groups of adrenergic blocking agents protect against the effects of elevated circulating catecholamines due to tumor.
alpha-adrenergic blockade should precede beta-receptor blockade in order to prevent severe hypertension due to unopposed (ß-receptor-mediated) a receptor mediated vasoconstriction.
alpha-adrenergic blockers--Phenoxybenzamine (Dibenzyline) or phentolamine (Regitine): used to control blood pressure prior to definitive surgical treatment
|
|
"Catecholamine-secreting pheochromocytoma of adrenal medulla gross. Note spherical enlargement of the adrenal medulla in this cross section of adrenal."
(c) 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center.
Hulyalkar, A. R., and Miller, E.D., Evaluation of the Hypertensive Patient in Principles and Practice of Anesthesiology (Longnecker, D.E., Tinker, J.H. Morgan, Jr., G. E., eds) Mosby, St. Louis, Mo., pp. 157-165, 1998.
Goldfien, A.,Adrenocorticosteroids and Adrenocortical Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 635-650.
Williams, G. H and Dluhy, R. G. , Diseases of the Adrenal Cortex, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 2035-2056.
![]()
Chronic Hypertension: Effects on Cardiac function
Early Cardiac problems associated with chronic hypertension
Diastolic relaxation abnormalities (may be subclinical)
Such diastolic dysfunction may manifest during surgical stress
Identification of diastolic dysfunction: echocardiography (Pulsed-wave Doppler)
Abnormal filling characteristic secondary to diminish left ventricular compliance (increasing ventricular wall stiffness)
Angina:
May occur in the absence of coronary vascular disease-- secondary to reduced coronary flow reserve
Transient myocardial ischemia may occur in the absence of coronary vascular disease in these patients {frequency = 50%}
Probable mechanism: abnormal coronary flow reserve {ischemia unrelated to left ventricular hypertrophy}
Left ventricular hypertrophy:
Reversibility: dependent on antihypertensive protocol
ACE inhibitors (e.g. captopril (Capoten)) or the calcium channel blocker verapamil (Isoptin, Calan): effective in reducing left ventricular hypertrophy
Other agents useful in left ventricular hypertrophy management:
Methyldopa (Aldomet)
Calcium channel blockers
Methyldopa (Aldomet)
Methyldopa (Aldomet) is a prodrug which is metabolized to the active agent, alpha-methylnorepinephrine.
Alpha-methylnorepinephrine acts in the brain, inhibiting adrenergic outflow from the brainstem. Inhibition of sympathetic outflow results in a decrease in blood pressure.
Methyldopa (Aldomet) produces no change in cardiac output in younger patients, but in older patients a decline in cardiac output results from reduced heart rate and stroke volume.
The reduction in stroke volume occurs due to increased venous pooling (decreased preload).
Since renal blood flow and function is maintained during methyldopa (Aldomet) treatment, methyldopa maybe valuable in managing hypertensive patients with renal insufficiency.
Calcium channel blockers
Calcium channel blockers are effective in treating hypertension because they reduce peripheral resistance.
Arteriolar vascular tone depends on free intracellular Ca2+ concentration.
Calcium channel blockers reduce transmembrane movement of Ca2+ , reduce the amount reaching intracellular sites and therefore reduce vascular smooth muscle tone.
All calcium channel blocks appear similarly effective for management of mild to moderate hypertension.
For low-renin hypertensive patients (elderly and African-American groups), Ca2+ channel blockers appear good choices for monotherapy (single drug) control.
Interactions with Anesthetics:
In anesthetized patients with preexisting left ventricular dysfunction--
verapamil (Isoptin, Calan) administration results in:
myocardial depression
reduced cardiac output
In patients with depressed left ventricular function, anesthetized with a volatile anesthetic,and undergoing open-chest surgery:
IV verapamil (Isoptin, Calan) or diltiazem (Cardiazem) further decreases ventricular function
In patients with preoperative cardiac conduction anomalies, treated with combined calcium channel blockers and beta-adrenergic receptor blockers: condition not associated with perioperative cardiac conduction abnormalities.
Interactions with neuromuscular-blocking drugs:
Calcium channel blockers potentiate depolarizing and nondepolarizing neuromuscular-blocking drug effects.
Similar to effects produced by "mycin" antibiotics in the presence of neuromuscular-blocking drugs (mycins:decrease acetylcholine release, e.g. gentamycin; tetracyclines - chelate calcium and decrease acetylcholine release)
Note that verapamil (Isoptin, Calan) possesses local anesthetic properties -- due to sodium channel blockade -- in effect which contributes to neuromuscular-blocking drug effect potentiation
Neuromuscular effects of verapamil (Isoptin, Calan): more likely to be evidenced in patients with reduced neuromuscular transmission margin of safety.
Neuromuscular-blockade antagonism: possibly impaired by reduced acetylcholine presynaptic release in the presence of a calcium channel blocker (presynaptic calcium influx is typically required for neurotransmitter release)
Risks associated with hypertensive cardiomyopathy and caused by myocardial ischemia {secondary to increased myocardial oxygen demand & reduced coronary flow reserve
Sudden Death
Ventricular Tachyarrhythmias
Abnormal ventricular compliance: major characteristic of hypertensive cardiomyopathy
Systolic function significantly limited in end-stage disease
Sequence in chronic hypertension:
Slow increase in left ventricular diastolic pressure (preload) leads to reactive fibrosis + hypertrophy [Increased hypertrophy reduces wall tension] which causes monocyte necrosis & reparative fibrosis {ACE inhibitors, e.g. lisinopril (Prinvivil, Zestril) may prevent reactive fibrosis but reparative fibrosis may be irreversible}
Ventricular hypertrophy -- significant wall thickening -- predisposes to ventricular arrhythmias
Hypertrophic & fibrotic myocardium associated with patients who have both diabetes & hypertension
![]()
ACE inhibitors
Angiotensin II, a potent vasoconstrictor, is produced by the action of angiotensin converting enzyme (ACE) on the substrate angiotensin I. Angiotensin II activity produces
a rapid pressor response
a slow pressor response and
vascular and cardiac hypertrophy and remodeling.
Antihypertensive effects of ACE inhibitors are due to the reduction in the amount of angiotensin II produced.
ACE inhibitors are efficacious in management of hypertension and have a favorable side effect profile.
ACE inhibitor are advantageous in management of diabetic patients by reducing the development of diabetic neuropathy and glomerulosclerosis.
ACE inhibitor are probably the antihypertensive drug of choice in treatment of hypertensive patient who have hypertrophic left ventricles.
Hypertensive patients who have ischemic heart disease with impaired left ventricular function also benefit from ACE inhibitor treatment.
ACE inhibitors reduce the normal aldosterone response to sodium loss (normally aldosterone opposes diuretic-induced sodium loss).
Therefore, the use of ACE inhibitors enhance the efficacy of diuretic treatment, allowing the use of lower diuretic dosages and improving control of hypertension.
If diuretics are administered at higher dosages in combination with ACE inhibitors significant and undesirable hypotensive reactions can occur with attendant excessive sodium loss.
Reduction in aldosterone production by ACE inhibitors also affects potassium levels.
The tendency is for potassium retention, which may be serious in patients with renal disease or if the patient is also taking potassium sparing diuretics, nonsteroidal anti-inflammatory agents or potassium supplements.
Hulyalkar, A. R., and Miller, E.D., Evaluation of the Hypertensive Patient in Principles and Practice of Anesthesiology (Longnecker, D.E., Tinker, J.H. Morgan, Jr., G. E., eds) Mosby, St. Louis, Mo., pp. 157-165, 1998. Stoelting, R.K., "Calcium Channel Blockers", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, p. 352-353.
Chronic Hypertension: CNS Effects
Hypertension & Stroke
Reduction in BP reduces stroke incidence
Hypertension predisposes to both ischemic & hemorrhagic stroke
Mechanism:
Hypertension damages vascular endothelium and in turn damaged endothelial cells promote:
(1) localized platelets aggregation
(2) thrombus formation and
(3) atheromatous plaque formation
Plaque formation reduces distal flow
Reduced vessel lumen dimension promotes turbulent blood flow which causes further injury
Smooth muscle media hypertrophy may weaken vascular integrity, increasing the likelihood of hemorrhagic stroke
Thrombotic Stroke
Acute thrombus at site of vessel narrowing
If a portion of the thrombus breaks off, distal vessel plugging results in embolic stroke
Predisposing factors for embolic stroke:
Cardiac arrhythmias
Atrial fibrillation (myocardial thrombus formation leading to cerebral emboli)
Hypertension predisposes the myocardium to arrhythmias
Infarcts secondary to hypotension, when BP falls below range of cerebral autoregulation
{Watershed infarcts = insufficient systemic blood-pressure for arterial blood to reach the end of vascular branches-- in particular poor cerebral cortical perfusion of a susceptible "watershed zone" between anterior & middle cerebral artery or middle & posterior cerebral artery may result in linear infarcts}
In the chronically hypertensive patient, a greater blood-pressure is required to maintain adequate cerebral perfusion compared to normal patients

Watershed Infarct image courtesy of the Digital Slice of Life Cooperative Project (http://medstat.med.utah.edu/kw/sol/sss/index.html)
![]()
Chronic Hypertension: Renal Effects
Chronic renal injury
Intimal hyperplasia
Afferent arteriolar sclerosis
Parenchymal fibrosis
secondary to reduced blood flow
Reduced renal function as a result of hypertension is characterized by a gradual degradation in renal function {benign nephrosclerosis}, leading to ultimately a rapid and irreversible renal failure {malignant nephrosclerosis}
Characteristics of benign nephrosclerosis
Renal dysfunction probably due to compensatory hyperfiltration by normal glomeruli
Increase glomerular pressure is associated with proteinuria
Hyperfiltration of certain glomeruli tends to induce sclerosis of otherwise normal glomeruli
Malignant Nephrosclerosis
Renal manifestation of malignant hypertension
If untreated (initially with IV vasodilators), malignant hypertension will precipitate renal failure within days-weeks
Treatment: typically IV vasodilators initially {e.g., nitroprusside sodium (Nipride), nitroglycerin}
Three renal histological changes associated with malignant hypertension
Arteriolar fibrinoid necrosis
Hyperplastic arteriosclerosis {arteriolar "onion-skinning"-note the vessel in the center of the image below}
Glomerulitis in the hypercellular glomerulus {screen position: 7 o'clock position below}
Description and image: courtesy of The Urbana Atlas of Pathology, use with permission
Malignant Nephrosclerosis Images


Image contribution by Saint Francis Hospital
Hulyalkar, A. R., and Miller, E.D., Evaluation of the Hypertensive Patient in Principles and Practice of Anesthesiology (Longnecker, D.E., Tinker, J.H. Morgan, Jr., G. E., eds) Mosby, St. Louis, Mo., pp. 157-165, 1998.
![]()
Chronic Hypertension: Perioperative Issues
Increased intraoperative blood-pressure lability in patients not receiving their antihypertensive medication: Recommendation -- administer medications on the day of surgery
-- Exception: possibly diuretics
Concerning elective surgery in patients with uncontrolled hypertension:
Surgery not associated with elevated incidence of perioperative cardiac morbidity if preoperative diastolic pressures were less than 110 mm Hg with careful perioperative blood-pressure monitoring
Preoperative approaches to the hypertensive patients:
Factors that increase risk of myocardial ischemia intraoperatively
uncontrolled hypertension or hypertension control solely with diuretics {compared to patients treated with atenolol (Tenormin)}
myocardial ischemia --most likely occurs during:
intubation
emergence from anesthesia
Intraoperative ischemia: primary association = tachycardia
Special considerations:
Diabetic patients with hypertension (noncardiac surgical procedures)
Predictors of postoperative ischemia/infarction
Preoperative cardiomegaly (demonstrate radiographically)
Previous myocardial infarction
Intraoperatively events predictive of postoperative for cardiovascular complications {renal/cardiac morbidity}
intraoperative hypotension (> 20 mm Hg decrease in mean arterial pressure (duration > 1 hour))
intraoperative hypertension (> 20 mm Hg increased in mean arterial pressure (duration > 15 minutes )) alternating with hypotension
Hypertension & organ transplantation
Occurs only in some patients
Hypertension increases the likelihood of renal failure in the transplanted kidney
Antihypertensive drug treatment: no improvement in graft survival
Post-organ transplantation hypertension probably due to immunosuppressive drugs:
Glucocorticoids -- Na+/H2O retention
Cyclosporine (Sandimmune, Neoral): increased sympathetic nervous system activity (suggested factor)
Perioperative issues in managing patients with cerebrovascular disease/hypertension:
Aneurysmal subarachnoid hemorrhage patients
Hypertension management may reduce re-bleeding
Hypertension management, however may increase cerebral infarction risk
Surgical intervention for aneurysmal subarachnoid hemorrhage in patients who have had hypertension: associated with increased seizure risk.
Diastolic pressures: best maintained in a range of 95-105 mm Hg
Post-operative control of BP & vasospasm-- nimodipine (Nimotop)
Nimodipine (Nimotop)
Overview
Highly lipid-soluble nefedipine analog
Ready access to the CNS -- reduces large cerebral arterial contraction
Clinical Use:
Cerebral Vasospasm:
Useful in preventing/reducing cerebral vasospasm associated with subarachnoid hemorrhage
Vasospasm -- mediated by calcium ion influx
Nimodipine (Nimotop) administered over a three week course (oral administration) results and decreased frequency of neurologic defects secondary to cerebral vasospasm in subarachnoid hemorrhage patients.
For comatose patients: deliver through nasogastric tube
Side effects/Concerns:
systemic hypotension (with excess nimodipine (Nimotop) effect)
possible increase in intracranial pressure -- especially in patients with decreased intracranial compliance
![]()
Hypertension & Anesthetic management
Elective surgery issues:
Cancel elective surgery if diastolic pressure > 115 mm Hg {until evaluation & treatment result in controlled pressure}
Emergency surgery issues:
Intraoperative management of BP with--
Short-acting parenteral drugs, e.g. nitroprusside sodium (Nipride), nitroglycerin, esmolol (Brevibloc)
Nitroprusside (Nipride)
Overview:nitroprusside (Nipride)
Direct-acting, nonselective peripheral vasodilator--NO mediated
Relaxation of arterial and venous vascular smooth muscle
Structure:
ferrous iron center complex with five cyanide moieties and a nitrosyl group (44% cyanide by weight)
Immediate onset of action
Short duration (requires continuous IV administration to maintain effect)
High-potency:
requires careful dosage titration
frequent systemic blood pressure monitoring -- often by intra-arterial catheter
Mechanism of Action: nitroprusside
nitroprusside interacts with oxyhemoglobin, forming methemoglobin with cyanide ion and nitric oxide (NO) release
NO activates guanylyl cyclase (in vascular smooth muscle);resulting in increased intracellular cGMP
cGMP inhibits calcium entry into vascular smooth muscle (may also increase calcium uptake by smooth endoplasmic reticulum): producing vasodilation
Mechanisms by which cGMP relaxes vascular smooth muscle remain to be elucidated. cGMP does, however, activate K+ channels (hyperpolarizing effect), activate a cGMP-dependent protein kinase, , decrease IP3, and inhibit calcium entry into the smooth muscle cells.
NO: active mediator responsible for direct nitroprusside vasodilating effect.
Note that organic nitrates (e.g. nitroglycerin) require thio-containing agents to generate NO
The reaction: nitroprusside interacts with oxyhemoglobin, leading to methemoglobin formation with cyanide ion and nitric oxide (NO) release produces an unstable nitroprusside radical
nitroprusside radicals decomposes releasing five cyanide ions (one cyanide reacts with methemoglobin to form cyanomethemoglobin)
remaining free cyanide ions (following reaction with hepatic & renal rhodanase) are converted to thiocyanate {thiosulfate donor: body sulfur stores are sufficient detoxifying about 50 milligrams nitroprusside})
Organ System Effects: Nitroprusside
Overview: Principal effects referable to:
Cardiovascular system
Cerebral blood flow
Hypoxic pulmonary vasoconstriction
Platelet aggregation
Cardiovascular Effects: Nitroprusside
Direct venous/arterial vasodilation; rapid decrease in systemic blood-pressure
Reduced systemic vascular resistance (arterial vasodilation; venous capacitance vessel vasodilation)
Positive inotropic & chronotropic responses: reflex-mediated secondary to hypotensive response
Net increase in cardiac output due to:
increase contractility
decreased left ventricular ejection impedance
Hypotensive response: associated with reduced renal function; renin release occurs (explains over shoot upon nitroprusside discontinuation {ACE inhibitor-sensitive, i.e. an ACE inhibitor will prevent this overshoot effect})
Nitroprusside: may worsen myocardial infarction damage due to "coronary steal", blood flow directed away from ischemic areas by arteriolar vasodilation
Cerebrovascular Effects:
Increased cerebral blood flow, volume.
with decreased intracranial compliance, increased intracranial pressure results
Generally, increases in intracranial pressure are most apparent when systemic mean arterial pressure decreases by less than 30%
if systemic mean arterial pressure decreases by > 30%, intracranial pressure decreases below the awake level.
Nitroprusside contraindicated in patients with known inadequate cerebral blood flow (e.g. high intracranial pressure; carotid artery stenosis)
Hypoxic Pulmonary Vasoconstriction
Nitroprusside infusion (and other vasodilators) causes decrease in PaO2
Mechanism: vasodilator-mediated reduction in hypoxic pulmonary vasoconstriction
Clinical Uses: -- nitroprusside (Nipride)
Control hypotension during anesthesia and surgery
Rapid, predictable vasodilation & decrease in BP allows a nearly bloodless surgical field, required in some operations: spine surgery, neurosurgery (also reduces transfusions)
Other drugs that might be chosen to produce controlled hypotension, nitroprusside is most likely to ensure adequate cerebral perfusion (mean arterial pressure's of 50-60 mm Hg can be maintained without apparent complications {in healthy patients})
The potential for cyanide toxicity can be diminished by:
Use of other cardiovascular depressant drugs which reduce nitroprusside requirements
These drugs include: volatile anesthetics, beta-adrenergic antagonists, calcium channel blockers; note that beta adrenergic antagonists may cause a decreased cardiac output-- a potential problem in patients with diminished the ventricular reserve.
Treatment of hypertensive emergencies
Acute & chronic heart failure
Reduction of afterload may be important for patients with CHF, mitral or aortic regurgitation, acute myocardial infarction with left ventricular failure
Role of nitroprusside in chronic, congestive heart failure -- advantageous because:
reduced ventricular ejection impedance (injection at lower end-diastolic volumes
preload reduction (secondary to blood pooling in venous capacitance vessels -- reflected in decreased ventricular and-diastolic volume)
Surgical indications:
Aortic surgery
reduction of proximal hypertension associated with aortic cross-clamping (thoracic aortic aneurysm, dissections, coarctations)
distal hypotension may occur (relative to clamp location)
Cardiac surgery necessitating cardiopulmonary bypass
Activation of renin-angiotensin system may cause systemic hypertension during cardiac surgery
Nitroprusside is effective in reducing such increases in BP
Following cardiopulmonary bypass {re-warming phase}, nitroprusside-mediated vasodilation facilitates heat delivery to tissues {reduces nasopharyngeal temperature decline after bypass}
Nitroprusside is effective in managing pulmonary hypertension after valve replacement
Pheochromocytoma resection
Vasodilators used for acute management of hypertensive crisis or malignant hypertension include sodium nitroprusside and diazoxide.
Nitroprusside sodium (Nipride) is the agent of choice-- advantages
Rapid onset
Effect diminishes rapidly upon drug discontinuation
May also be used (rapid injection) to reduce systemic blood-pressure associated with direct laryngoscopic tracheal intubation
Administered by a continuously variable rate i.v. infusion pump, precise blood pressure control can be obtained.
Nitroprusside sodium (Nipride), a nitrovasodilator, is metabolized by smooth muscle cells to nitric oxide which dilates both arterioles and venules.
If patients have controlled hypertension, anxiolytics may be appropriate {preoperative medication}
diazepam (Valium); oral
midazolam (Versed); intramuscular
Patients managed with diuretic drugs for hypertension:
Diuretics not given on same day of surgery
Patients may be volume depleted
Patients whose hypertension is managed with diuretics may have to be dehydrated prior to the surgical procedure {remaining antihypertensive medications, i.e. non-diuretics should be administered on the surgical day.}
Management of hemodynamic variability during anesthetic induction/emergence:
Reduction of variability
Esmolol (Brevibloc) {short-acting ß-receptor blockers}
Effective in management of sinus tachyarrhythmias & hypertension in cardiac & noncardiac surgical patients
Lidocaine (Xylocaine)
Nicardipine (Cardene)-management of perioperative hypertension
Choice of anesthetic for maintenance:
Any agent reasonable except for ketamine (Ketalar)
Ketamine (Ketalar) associated with hypertension, increased intracranial pressure & tachycardia
Intraoperative management of acute blood pressure increases:
Prior to antihypertensive drug administration, rule out:
hypoxemia
hypercarbia
inadequate anesthetic depth
Hulyalkar, A. R., and Miller, E.D., Evaluation of the Hypertensive Patient in Principles and Practice of Anesthesiology (Longnecker, D.E., Tinker, J.H. Morgan, Jr., G. E., eds) Mosby, St. Louis, Mo., pp. 157-165, 1998. Stoelting, R.K., "Calcium Channel Blockers", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, p. 350; Stoelting, R.K., "Antihypertensive Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 302-312;and "Peripheral Vasodilators", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 315-322.
![]()
Pharmacological Management of Hypertension
Non-pharmacological interventions: Diet, Stress reduction, regular aerobic exercise, weight reduction as needed, control of other risk factors (blood lipids, smoking)
Diet involves several aspects that may include:
reduction of sodium intake
caloric restriction for obese patients
restriction of cholesterol and saturated fat intake.
Pharmacological Management
Pharmacological interventions in essential hypertension management is based on selecting often a single initial agent and evaluation of patient response. Unless clinical conditions require immediate reduction in blood pressure as in hypertensive crisis, the first step may involve the use of :
a calcium channel blocker or beta-blocker, or angiotensin converting enzyme inhibitor (ACE inhibitor). The specific clinical presentation may favor one choice over another.
For example, in patients with asthma or chronic obstructive pulmonary disease (COPD) the use of a beta-adrenoceptor antagonist may be contraindicated since agents belonging to this drug class may cause bronchoconstriction.
Alternatively, a patient with marginal left ventricular function, predisposed to congestive heart failure, may not tolerate beta-blockers or calcium channel blockers because both mediate a negative inotropic effect (reduced myocardial contractility). At this step, beginning with a low dosage first is usually appropriate.
Thiazide diuretics has been often used in the past as the first drug given.
Thiazides may be added if adequate blood pressure control by a calcium-channel blocker, a beta-blocker, or an ACE inhibitor has not been achieved.
Thiazides promote potassium loss and increases in serum lipids.
If control has not been achieve with the above drugs at optimal dosages, a antiadrenergic drug may be added (such as a central or peripheral-acting sympatholyic). Peripheral vasodilators may also be used at this stage.
The general idea is to use a single agent before progressing to multiple agents and then stronger multiple drugs. Often the side-effect profile becomes less favorable as more and stronger drugs are added.
|
Note the progression of antihypertensive medication
At each step dosages are reviewed and if the patient's hypertension is controlled then therapy may be continued with review for possible removal of medication. Figure adapted from Harrison's "Principles of Internal Medicine, Thirteenth Edition, p. 1128
|
 |
For hypertensive emergencies/crises sodium nitroprusside is the agent of first choice.
Hypertensive Crisis
Definition: diastolic pressure > 130 mm Hg
Malignant hypertension (evidence of end-organ damage): Medical emergency-requires immediate treatment
Malignant hypertension requires the use of parenteral agents with intra-arterial BP monitoring
Without end-organ damage present, oral or sublingual antihypertensive drugs might be used
Factors causing a rapid increase in BP:
Elevation of intracranial pressure (increased blood pressure allows maintenance of cerebral perfusion)
Neurological causes of hypertensive crisis:
intracranial hemorrhage
head trauma
CNS tumor
thromboembolic stroke
subarachnoid hemorrhage
Cautious management for patients with hypertensive crisis --hypertensive drugs given in severe hypertension (> 200/130 mm Hg)
Nitroprusside sodium (Nipride) -- careful administration {may increase intracranial pressure and patients with reduced intracranial compliance)
Beta adrenergic receptor blocking drugs: Not recommended; may cause cerebral vasospasm
Centrally acting drugs {e.g., clonidine (Catapres); methyldopa (Aldomet)}: not recommended for hypertensive crisis as a result of neurological cause
Hemorrhage, Subarachnoid, Hypertensive Vessels (left); Hemorrhage, Intraventricular Medulla, Extension into Lateral Recess and Subarachnoid Space (right): Courtesy of Digital Slice of Life (http://medstat.med.utah.edu/kw/sol/sss/index.html)
|
|
|
Cardiovascular causes of hypertensive crisis:
Myocardial infarction
Dissecting aortic aneurysm
Therapeutic Objectives:
20%-25% reduction in diastolic or pressure (100 mm Hg)
For dissecting aortic aneurysm cases, reducing the arterial pressure rate of rise may prevent aneurysmal rupture-{nitroprusside sodium (Nipride) + ß-adrenoceptor blocker is a useful combination}
In myocardial infarction, maintenance of coronary perfusion is critical as systemic pressure is reduced-Useful drugs include:
nitroglycerin
calcium channel blockers
ß adrenoceptor blockers
Renal causes of hypertensive crisis:
Renal artery stenosis
Parenchymal renal disease
Therapeutic objectives:
maintain renal perfusion while decreasing BP to prevent fibrinoid necrosis
Useful drugs:
Nitroprusside sodium (Nipride)
Calcium channel blockers
Other interventions:
the combination of renal failure & malignant hypertension may require dialysis
Special cautions:
Thiocyanate levels must be monitored when nitroprusside sodium (Nipride) is used at high doses for extended periods of time
ACE inhibitors should not be used if bilateral renal artery stenosis suspected/confirmed:
Mechanism -- glomerular filtration is dependent on post-glomerular arteriolar constriction, which is maintained by angiotensin II. Renal failure can be induced if post-glomerular arterial constriction is lost.
Other causes of hypertensive crisis:
Ingestion of tyramine-rich foods in patients taking MAO inhibitors
Preeclampsia
Recreational drug use
Hyperautonomic syndromes {chronic smoke or dysfunction}
Pheochromocytoma-massive release of endogenous catecholamines by tumor.
 |
 |
Hulyalkar, A. R., and Miller, E.D., Evaluation of the Hypertensive Patient in Principles and Practice of Anesthesiology (Longnecker, D.E., Tinker, J.H. Morgan, Jr., G. E., eds) Mosby, St. Louis, Mo., pp. 157-165, 1998.
|
|
|
References
Hoffman B B "Therapy of Hypertension" in Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11th Edition, (Brunton LL, Lazo JS and Parker KL, eds) McGraw-Hill Medical Publishing Divsion, NewYork, 2006.
Sutters M "Systemic Hypertension", Chapter 11 in Current Medical Diagnosis and Treatment 2010, 49th eds (McPhee SJ, Papadakis MA eds, Gonzales, Zeiger R, Online eds,) The McGraw-Hill Companies, New York, 2010
Sutters M "Systemic Hypertension", Chapter 11 in Current Medical Diagnosis and Treatment 2008, 47th eds (McPhee SJ, Papadakis MA eds, Tierney LM, Jr, senior editor) The McGraw-Hill Companies, New York, 2008.