|
|
Chapter 13: Pain Management: Opioids
Opioids
"Narcotic" is a somewhat imprecise term because it suggests "narcosis", which is indicated of a somnolent state or "sleepy" state.
"Opioid analgesic" and therefore is a more appropriate term emphasizing the clinically important analgesic property which is the pharmacological property of importance in the therapeutic application of these agents.
Accordingly, opioids are used without the expectation that they themselves will cause sleep. However, opioids are frequently used in combination with anesthetics and in that context anesthesia may be obtained requiring less anesthetic.
All natural/semisynthetic opium alkaloid derivatives, synthetic agents, and other agents whose opioid-like effects are blocked by classical opioid antagonists, such as naloxone (Narcan) or naltrexone (ReVia).
Opium -- from the opium poppy (Papaver somniferum). Opium is obtained following drying the milky juice from unripe seed pod.
Opium has a characteristic odor and bitter-taste with its chief active ingredient being morphine.
Also present are codeine, thebaine (a non-analgesic agent), noscapine and papaverine, a non-analgesic vasodilator.
Tincture of opium is called laudanum. (Tincture is a generic term which refers to an alcohol solution of a nonvolatile medicine. Paregoric is a mixture of opium, alcohol, and camphor.)
An opioid full agonist activates opioid receptors, exhibiting high efficacy.
High efficacy refers to a maximal opioid effect, typically pain relief.
Full agonists may have comparable efficacies with the differing potencies meaning that different amounts of one drug compared to another may have to be given in order to achieve a maximal effect.
A partial agonist may itself cause agonist effects but because they can displace through competitive action a full agonist from its receptor, the net effect is a reduction in drug effect. As a result, a partial agonist, depending on circumstance, can act as either in agonist or an antagonist.
Antagonists: Pharmacological effects of opioids are mediated by interaction with differing opioids receptor types.
Most of the pharmacological effects as well as side effects, at least respiratory depression, are mediated by opioid-mu receptor interactions.
These agonist-mediated effects may be blocked by competitive inhibition by agents that occupy the the same receptor by do not activate it, yet prevent activation by agonists.
Furthermore, an opioid might be an agonist at one receptor subtype, but only a partial agonist or even in antagonist at another subtype.
Examples:
Naloxone (Narcan): pure antagonist: no effects normally associated with agonist binding
Morphine: full agonist at mu receptor
Codeine: partial or "weak" agonist -- less than maximal theoretical effect despite complete receptor saturation
Nalbuphine (Nubain) : agonist that one opioid receptor; antagonist at another
Partial agonist/antagonist characteristics: replacement of methyl moiety on the nitrogen atom with larger substituents:
Allyl substitution-- nalorphine and naloxone
Substitutions at the C3 and C6 morphine hydroxyl groups (see below)
Pharmacokinetic properties altered
Methyl substitution at C3 reduces first-pass hepatic metabolism by glucuronide conjugation: -- as a consequence codeine and oxycodone have a higher oral: parenteral potency

|
|
|
|
|
|
|
|
|
Acetylation of both morphine hydroxyls = heroin {more rapid access across the blood-brain barrier compared morphine}; in the brain heroin is rapidly hydrolyzed to monoacetylmorphine and morphine
Endogenous Opioid Peptides: The rationale for endogenous opioid peptides came from the idea that opioid receptors are probably present in the body for the purpose of interacting with endogenous or naturally occurring substances. As a consequence, research proceeded to attempt identification of these naturally occurring substances now known as ß-endorphins and related peptides.
Morphine (and related agents) cause analgesia by acting at the brain regions containing peptides which have opioid-like properties
Endogenous substances = endogenous opioid peptides
Previous used term "endorphin" now refers to ß-endorphins and related peptides derived from the precursor: prepro-opiomelanocortin
Most widely distributed opioid analgesic peptides:
pentapeptides
methionine-enkephalin (met-enkephalin)
leucine-enkephalin (leu-enkephalin)
Three major precursor proteins:
Prepro-opiomelanocortin (POMC) {contains}:
met-enkephalin sequence
ß-endorphin sequence
some nonopioid peptides:
ACTH
ß-lipotropin
melanocyte-stimulating hormone
Preproenkephalin (proenkephalin A ) {contains}:
six copies of met-enkephalin
one copy of leu-enkephalin
Preprodynorphin (proenkephalin B) {contains-- active peptides containing the leu-enkephalin sequence}:
dynorphin A
dynorphin B
a and ß neoendorphin
Endogenous opioid precursors which are localized at pain modulation brain regions are probably released during stress, including pain or pain anticipation.
Also, precursor molecules for endogenous opioids are localized in adrenal medulla and gut neural plexuses
Opioid analgesics are generally well absorbed by cutaneous/intramuscular/mucosal surfaces
Transdermal fentanyl represents an important Route of Administration
Gastrointestinal absorption:
Some opioids-- subject to first-pass effects:
Codeine; oxycodone -- high oral: parenteral potency (protected from conjugation by substitution on C3 aromatic hydroxyl)
Various extent of plasma protein binding
Highest concentrations in tissues will be a function of perfusion
Skeletal muscle represents the largest reservoir
For highly lipophilic opioids (e.g. fentanyl), there is significant concentration in adipose tissue
Blood Brain Barrier:
Amphoteric agents (possessing both an acidic and basic group, e.g. morphine {phenolic hydroxyl at C3}: greatest difficulty for brain entry
Other substitutions that C3 improve blood-brain barrier penetration: e.g., heroin, codeine
Neonatal considerations: neonates lack the blood-brain barrier:
Placental opioid transfer (uses in obstetric analgesia) can result in depressed respiration in the newborn.
Conversion to polar metabolites; renal excretion
Opioids with hydroxyl groups are likely conjugated with glucuronic acid
Examples: morphine, levorphanol (Levo-dromoran)
Morphine-6-glucuronide: analgesic potency (perhaps > parent compound morphine)
In patients with compromised renal function, accumulation of metabolites occurs which prolongs analgesia
Esters-type opioids are: hydrolyzed by tissue esterases:
Examples: heroin, remifentanil (short duration of action)
N-demethylation: minor pathway
Accumulation of demethylated meperidine (Demerol) metabolite, normeperidine:
patients with decreased renal function or on high dosages: CNS excitatory effects:
seizures (more likely in children)
Oxidative metabolism (hepatic) primary route of phenylpiperidine opioid metabolism:
fentanyl (Sublimaze)
alfentanil (Alfenta)
sufentanil (Sufenta)
Polar metabolites -- renal; small amounts excreted unchanged
Glucuronide conjugates -- bile (enterohepatic circulation minor)
|
Way, W.L., Fields, H.L. and Way, E. L. Opioid Analgesics and Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 496-515. |
|
Coda, B.A. Opioids, In Clinical Anesthesia, 3rd Edition (Barash, P.G., Cullen, B.F. and Stoelting, R.K.,eds) Lippincott-Ravin Publishers, Philadelphia, New York, 1997, pp 329-358. |
|
Schuckit, M.A. and Segal D.S., Opioid Drug Abuse and Dependence, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 2508-2512. |
![]()
Analgesia: specific receptor binding -- localization:
spinal cord
brain
Mu (m)
Delta (d)
Kappa (k)
General Opioid Receptor Characteristics:
G protein coupled receptor family
Significant amino acid sequence homology
Each-receptor: subtypes
Mu1, Mu2
Delta1, Delta2
Kappa1, Kappa2
Receptor types and physiological effects:
Mu (m) :Analgesia, euphoria, respiratory depression, physiological dependence
Most opioid analgesics: act at the mu receptor
Delta (d) and Kappa (k): Spinal analgesia
Drugs/endogenous opioids: Receptor- type affinity
morphine -- (m)
pentazocine -- (k) some (m)
endogenous opioid peptides:
leu-enkephalin --(d)
dynorphin --(k)
|
Drug |
Mu (m) |
Delta (d) |
Kappa (k) |
|
Opioid Peptides |
|||
|
Enkephalins |
Antagonist |
Agonist |
|
|
beta-endorphin |
Agonist |
Agonist |
|
|
Dynorphin |
Weak Agonist |
Agonist |
|
|
Agonists |
|||
|
Codeine |
Weak Agonist |
Weak Agonist |
|
|
etorphine |
Agonist |
Agonist |
Agonist |
|
fentanyl (Sublimaze) |
Agonist |
||
|
meperidine (Demerol) |
Agonist |
||
|
methadone (Dolophine) |
Agonist |
||
|
Morphine |
Agonist |
Weak Agonist |
|
|
Agonist-antagonists |
|||
|
Buprenorphine |
Partial Agonist |
||
|
dezocine (Dalgan) |
Partial Agonist |
Agonist |
|
|
nalbuphine (Nubain) |
Antagonist |
Agonist |
|
|
pentazocine (Talwain) |
Antagonist or Partial Agonist |
Agonist |
|
|
Antagonist: naloxone (Narcan) |
Antagonist |
Antagonist |
Antagonist |
Opioids: G protein linked-- affecting
Ion channel state
Intracellular Ca2+ levels
Protein phosphorylations states
Two well-defined opioid actions:
Reduce neurotransmitter release; by closing a voltage-gated Ca2+ channel on presynaptic neuronal terminals Or
Inhibit postsynaptic neurons (hyperpolarization) by increasing and K+ channel conductance
Spinal cord presynaptic sites:
Reduced transmitter released-- affects acetylcholine, norepinephrine, glutamate, serotonin, substance P
|
|
Serotonin, bradykinin, histamine, prostaglandins, substance P (sP) , and various ions (ie, H+ or K+)--the biochemical mediators released as a result of tissue injury--have been implicated in nociceptive activation and sensitization (hyperalgesia).
Hyperalgesia results in enhancement of spontaneous pain via a reduction in pain threshold and a lengthening in duration of nociceptor response to stimuli.
PGE1, PGE2, and PGF2a, are the most potent prostaglandins to produce these sensitization effects.
Substance P, synthesized by cells of the spinal ganglia, has been identified at the peripheral terminal of unmyelinated primary afferent fibers.
This putative neurotransmitter may play a role in the propagation of visceral nociceptive pain from the gastrointestinal (GI) tract, ureters, and urinary bladder.
In addition, to sP, other potential nociceptive transmitters include glutamate, aspartate, somatostatin, cholecystokinin, and vasoactive intestinal polypeptide.
courtesy of Roxane Pain Institute used with permission
|
Way, W.L., Fields, H.L. and Way, E. L. Opioid Analgesics and Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 496-515. |
|
Schuckit, M.A. and Segal D.S., Opioid Drug Abuse and Dependence, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 2508-2512. |
|
Coda, B.A. Opioids, In Clinical Anesthesia, 3rd Edition (Barash, P.G., Cullen, B.F. and Stoelting, R.K.,eds) Lippincott-Ravin Publishers, Philadelphia, New York, 1997, pp 329-358. |
Primary afferents to pain transmission neurons
Opioid agonists:
Inhibit excitatory transmitters release from these primary afferents
Inhibit dorsal horn pain transmission neurons
Clinical application: directed administration of opioid agonists allow regional analgesia which minimizes CNS side effects
Systemic Opioid Administration:
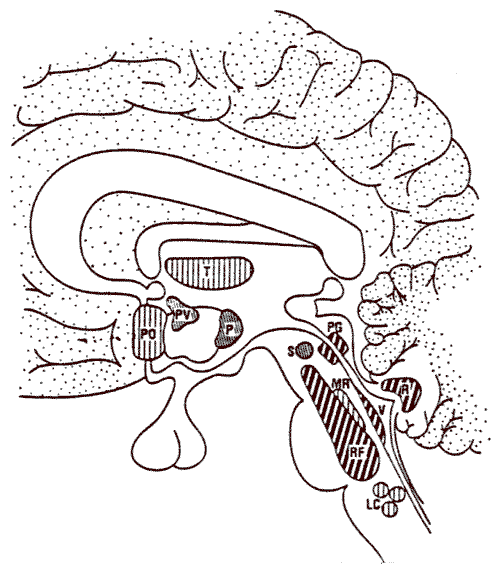
Important opioid binding sites in descending pathways
Rostral ventral medulla
Locus ceruleus (see below)
Midbrain periaqueductal gray
Administration of exogenous opioids promotes release of endogenous opioids
 |
|
Repeated opioid administration results in a gradual loss of effect, e.g. tolerance
Physical Dependence = physiological withdrawal symptoms (abstinence syndrome) if an antagonist is administered or the agonist is stopped.
Tolerance is not developed equally to all opioid effects.
| High | Intermediate | Limited/None |
| analgesia | bradycardia | miosis |
| euphoria, dysphoria | constipation | |
| mental clouding | convulsions | |
| sedation | antagonist actions | |
| respiratory depression | ||
| antidiuresis | ||
| nausea/vomiting | ||
| cough suppression |
adapted from Figure 31-4: Way, W.L., Fields, H.L. and Way, E. L. Opioid Analgesics and Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, p. 505.
| rhinorrhea | lacrimation | chills | hyperventilation | muscular aches | vomiting |
| anxiety | diarrhea | hostility | piloerection | yawning | hyperventilation |
|
|
|
|
|