|
|
Anesthesia Pharmacology Chapter 22: Adrenocorticosteroid Pharmacology
Adrenocorticosteroids and Adrenocortical Antagonists
Natural adrenocortical hormones:
steroid molecules synthesized in released by the adrenal cortex
Clinical Uses:
diagnosis of adrenal function
treatment of adrenal function disorders
treatment of inflammatory/immunological disorders (at higher doses)
Control over adrenocorticosteroid secretion:
pituitary corticotropin (ACTH) release
Angiotensin modulation of aldosterone secretion
Corticosteroids with:
androgenic activity
estrogenic activity
Major androgen: dehydroepiandrosterone (DHEA)
quantitatively -- DHEA
DHEA and androstenedione )very weak androgens
small amount of testosterone, secreted by the adrenals, maybe more important
adrenal androgens: testosterone and androstenedione may be converted to estrone by non-endocrine tissue:
Major endogenous estrogen source in women after menopause
Glucocorticoids (naturally occurring; cortisol -- hydrocortisone)
major glucocorticoid: cortisol
precursor: cholesterol
site of adrenal cortisol synthesis:
zona fasciculata
zona reticularis
cortisol release modulated by ACTH
Release rate of cortisol controlled by circadian rhythm affected by ACTH pulses
75% of cortisol bound to plasma proteins
corticosteroid-binding globulin (CBG) -- alpha2 globulin;
cortisol also bound to serum albumin
free cortisol plasma concentrations rise rapidly if CBG binding capacity is exceeded.
Factors that change plasma CBG concentration
![]() pregnancy
pregnancy
![]() estrogen
administration (increased
hepatic synthesis)
estrogen
administration (increased
hepatic synthesis)
![]() hyperthyroidism
hyperthyroidism
![]() hypothyroidism
hypothyroidism
![]() protein
deficiency
protein
deficiency
![]() diminished
synthetic capability
(genetics)
diminished
synthetic capability
(genetics)
cortisol half-life: about 60-90 minutes
Factors that increase cortisol half-life
stress
hypothyroidism
liver disease
large dosage of hydrocortisone administration
20% converted to cortisone (by renal/other tissues with mineralocorticoid receptors) -- catalyzed by 11-hydroxysteroid dehydrogenase
Cortisol and cortisone inactivated in the liver by conversion (3-hydroxysteroid dehydrogenase catalyzed) to:
tetrahydrocortisol
tetrahydrocortisone
Other metabolites: cortol, cortolone
Some metabolites ultimately excreted in the urine as 11-oxy, 17-ketosteroids
Some metabolites undergo hepatic conjugation to form glucuronic acid or sulfate derivatives
Glucocorticoid action through glucocorticoid receptors
member of receptor superfamily that includes:
steroid receptors
thyroid receptors
other receptors (many with unknown function -- "orphan receptors"
Receptors bound to heat shock proteins (Hsp/hsp90)
Free glucocorticoid hormone enters the cell
binds to the receptor, inducing a conformational change
receptor dissociates from heat shock proteins
hormone-receptor complex associate to form homodimers
homodimers actively transported to the nucleus
homodimers mind to glucocorticoid receptor elements (GREs) of target genes
Genomic effects -- protein synthesized; Indirect mediation of some genomic effects by paracrine influences of hormone-regulated cytokines on nearby cells
Some physiological effects occur to rapidly to be accounted for by gene transcription/ protein synthesis:
feedback suppression of pituitary ACTH (unknown mechanism)
Physiological effects of glucocorticoids:
Major metabolic effects: due to direct cellular action
Some effects:secondary to homeostatic insulin and glucagon responses
Physiological responses modulated by glucocorticoids ("permissive" effects)
catecholamine vascular/bronchial smooth muscle response:
diminished in the absence of cortisol
restored by physiological amounts of cortisol
catecholamine-induced lipolytic adipocytes response:
reduced in the absence of glucocorticoids (unknown mechanism)
Glucocorticoids: stimulate and are required for:
gluconeogenesis (fasted state, diabetes);
increasing hepatic and renal amino acid uptake
increase gluconeogenic enzyme activity
Simulation of glycogen synthase
Increase glucose production from protein-- stimulating insulin release
inhibit glucose uptake promoting increased lipolysis
counteracted by enhanced insulin secretion which stimulates lipogenesis
net effect: fat deposition
Glucocorticoid effects most prominent in the fasting state, through:
stimulation:gluconeogenesis
stimulation: amino acid release from muscle (catabolism)
inhibition: peripheral glucose uptake
stimulation: lipolysis
promotion of catabolism:
lymphoid tissue
connective tissue
muscle
fat
skin
High (supraphysiologic) glucocorticoid levels cause:
decreased muscle mass, weakness
reduced growth in children (not prevented by growth hormone)
Catabolic effects on bone:
osteoporosis in Cushing's syndrome
major limitation in long-term use
Anti-inflammatory/Immunosuppressive Effects:
Reduce inflammation --
Leukocyte-mediated; reduced leukocyte infiltration
glucocorticoid inhibition of interactions involving cell adhesion molecules (especially on endothelial cells)
Following glucocorticoid administration:
neutrophils levels are increased, but a decrease is noted in lymphocytes (T and B cells, monocytes, eosinophils, basophils-- movement from vasculature to lymphoid tissue)
Glucocorticoids inhibit:
leukocyte and tissue macrophage function
reduced antigenic and mitogenic responsiveness
Macrophage effects:
decreased interferon-gamma, interleukin 1, pyrogen, collagenase, elastase, tumor necrosis factor, plasminogen activator
Lymphocyte effects:
decreased interleukin 2
Reduction of prostaglandin and leukotriene synthesis (resulting from phospholipase A2 activation)
Reduction of cyclooxygenase in inflammatory cells (reducing prostaglandin synthesis)
of the two isoforms of cyclooxygenase (COX1 and COX-II), glucocorticoids inhibit COX-II gene expression.
Glucocorticoids decrease capillary permeability by:
reducing kinin activity
reducing bacterial endotoxin activity
reducing basophils histamine release
adrenal-insufficiency: EEG changes (slowing of alpha rhythms)
increased levels: behavioral changes;
decreased pituitary release of ACTH and beta-lipotropin
decreased TSH and FSH secretion
increased excess acid/pepsin production (large doses)
increased fat absorption
in addition: effective vitamin D on calcium absorption
increased platelet production, erythrocyte production
without adequate cortisol: renal function -- impaired
glucocorticoids important in tissue development (structure/functional changes in the lung)
Source:
Synthesized from cholic acid (from cattle sources) or
Synthesized from steroid sapogenins (diosgenin) -- plants
Disposition:
oral administration; complete absorption
metabolized similar to endogenous steroids
molecular alterations given rise to differences in:
affinity for mineralocorticoid or glucocorticoid receptors
extent of protein binding
stability
spectrum of metabolic products
prodrugs may be used (prednisone is converted to prednisolone)
Similar to those of cortisol (endogenous agents)
|
Drug |
Anti-inflammatory |
Salt-retaining |
Dosage Forms |
|
Short/medium-acting glucocorticoid |
|
hydrocortisone (cortisol) |
1 |
1 |
oral, injectable, topical |
|
cortisone (Cortone) |
0.8 |
0.8 |
oral, injectable, topical |
|
prednisone (Deltasone) |
4 |
0.3 |
oral |
|
prednisolone (Prelone) |
5 |
0.3 |
oral, injectable, topical |
|
methylprednisolone (Solu-Medrol) |
5 |
0 |
oral, injectable, topical |
|
Intermediate-acting glucocorticoid |
|
triamcinolone (Aristocort) |
5 |
0 |
oral, injectable, topical |
|
fluprednisolone |
15 |
0 |
oral |
|
Long-acting glucocorticoid |
|
betamethasone (Celestone) |
25-40 |
0 |
oral, injectable, topical |
|
dexamethasone (Decadron) |
30 |
0 |
oral, injectable, topical |
|
Drug |
Anti-inflammatory |
Salt-retaining |
Dosage Forms |
|
Mineralocorticoids |
|
fludrocortisone (Florinef) |
10 |
250 |
oral, injectable, topical |
|
desoxycorticosterone acetate |
0 |
20 |
injectable, pellets |
|
Adapted from Table 39-1: Goldfien, A.,Adrenocorticosteroids and Adrenocortical Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, p. 640. |
Primary Adrenocortical insufficiency (Addison's Disease):
Rare; may occur at any age; affects both sexes with equal frequency
Addison's disease is caused by progressive destruction of the adrenals (> 90% must be destroyed before symptoms of adrenal insufficiency appear)
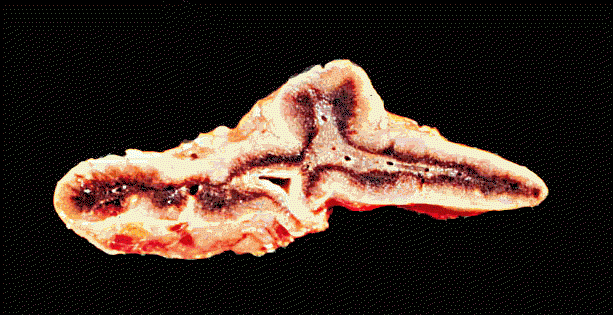
"Atrophic adrenal, gross, in chronic adrenocortical insufficiency "; Ó 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center.For more information concerning endocrine pathology
adrenal: common site for chronic granulomatous diseases, e.g.:
tuberculosis (mainly)
histoplasmosis
coccidiodomycosis
cryptococcosis
Adrenoleukodystrophy: significant demyelination -- early death and children
Adrenomyeloneuropathy: mixed motor/sensory neuropathy with spastic paraplegia -- (adults)
AIDS patients-- Higher likelihood of adrenal-insufficiency because:
cytomegalovirus frequently involves the adrenal glands:
CMV necrotizing adrenalitis
involvement with Mycobacterium avium-intracellulare, Cryptococcus, and Kaposi sarcoma
note: in interpreting results from adrenal function test in AIDS patients that certain medications may potentiates adrenal insufficiency including:
opiates
rifampin
phenytoin (Dilantin)
ketoconazole (Nizoral)
in early cases, tuberculosis caused 70%-90% of cases
Most frequent cause today is idiopathic atrophy.
autoimmune mechanism -- most likely
half of patients: circulating adrenal antibodies
adrenal antigens, e.g.: P450c21
some antibodies may cause adrenal destruction
other antibodies may cause adrenal insufficiency by inhibiting ACTH binding
some individuals also have antibodies to thyroid, parathyroid, and/or gonadal tissue
Increased likelihood of:
chronic lymphocytic thyroiditis
premature ovarian failure
Type I diabetes mellitus
hypothyroidism
hyperthyroidism
Presence of two or more autoimmune endocrine disorders in the same patient: polyglandular autoimmune syndrome
|
fatigue (99%) |
weakness (99%) |
anorexia (90%) |
|
nausea (90%) |
vomiting (90%) |
weight loss (97%) |
|
cutaneous/mucosal pigmentation (99%, 82%) |
hypotension (87%,<than 110/70 mmHg) |
hypoglycemia (occasionally) |
Asthenia, "Cardinal symptom":
severe fatigue, impairment; bed rest may be necessary
diffuse brown, tan, bronze darkening at elbows, hand creases
may include bluish-black mucosal membrane patches
Arterial hypotension with orthostatic component
Gastrointestinal disturbances: frequent presenting symptom
Primary Adrenocortical insufficiency: Laboratory Findings and Diagnostic Testing.
initially:steroid output normal; but adrenal reserve reduced
ACTH-adrenal stimulation: produces some normal cortisol increase or no increase
more advanced disease: (more adrenal destruction)
serum sodium, bicarbonate, chloride: reduced
decreased serum sodium: due to excessive urinary loss (secondary to aldosterone deficiency) and movement into intracellular compartments
extravascular sodium loss -- depleting extracellular fluid; promotes hypotension; elevated plasma angiotensin II and vasopressin promote hyponatremia by reducing free water clearance
serum potassium: elevated
Hyperkalemia due to:
aldosterone deficiency
acidosis
impaired glomerular filtration
Based on ACTH stimulation testing: evaluation of adrenal steroid production reserve capacity
Severe adrenal insufficiency: rate of cortisol secretion significantly reduced; low to absent 24 urine cortisol levelto
Mild adrenal insufficiency (decreased adrenal reserve)
Aldosterone secretion: low-- causing:
salt wasting
increased plasma renin
|
Treatment of Primary Adrenocortical Deficiency (Addison's Disease)
correction of both glucocorticoid and mineralocorticoid deficiency
Therapeutic Mainstay: cortisol
Treatment complications: rare; except for gastritis
Mineralocorticoid component: fludrocortisone
adequacy assessed by serum electrolyte and blood pressure measurements
blood pressure: normal; no orthostatic effects
serum sodium, potassium, creatinine, blood urea nitrogen levels: normal
Treatment complications:
hypokalemia
hypertension
cardiac enlargement
congestive heart failure (secondary to sodium retention)
During illness (especially if fever is present): hydrocortisone dosage should be increased (doubled)
Supplemental glucocorticoid dosage before:
surgery
dental extraction
Supplemental fludrocortisone plus salt upon:
strenuous exercise with sweating
during very hot weather
gastrointestinal upsets
Acute Adrenocortical Insufficiency:
May occur are due to:
rapid intensification of chronic adrenal insufficiency
precipitated by sepsis or surgical stress
acute hemorrhagic adrenal gland destruction in a previously healthy individual
in children: associated with Pseudomonas septicemia or meningiococcemia
in adults: associated with anticoagulant treatment/coagulation disorder
Most frequent cause of acute adrenal insufficiency:
rapid withdrawal of steroids from patients who have adrenal atrophy following prolonged chronic steroid administration
Other causes:
patients with congenital adrenal hyperplasia or with decreased adrenocortical reserve when:
they are given drugs that inhibit steroid synthesis, e.g. mitotane (Lysodren), ketoconazole (Nizoral) or
they are given drugs that increase steroid metabolism, e.g. phenytoin (Dilantin), rifampin (Rimactane)
Long-term survival: dependent on prevention and proper treatment of adrenal crisis:
Prevention of crisis: infection, trauma, gastrointestinal upsets, other stresses: require immediate increase in administered hormone. Otherwise, symptoms may intensify --
nausea
vomiting
abdominal pain
lethargy, somnolence
hypovolemic vascular collapse
Treatment: based on replacing glucocorticoids and sodium/water deficits
intravenous 5% glucose infusion (in normal saline)
initiated with IV bolus of 100 mg hydrocortisone, followed by continuous hydrocortisone (Cortef, Solu-Cortef) infusion (10 mg/h)
Management of hypotension requires glucocorticoid replacement and correction of sodium and water deficit
Vasoconstrictive agents (dopamine) may be required in some extreme cases
Mineralocorticoid supplementation may be required (full mineralocorticoid effect will accompany the 100 mg hydrocortisone infusion)
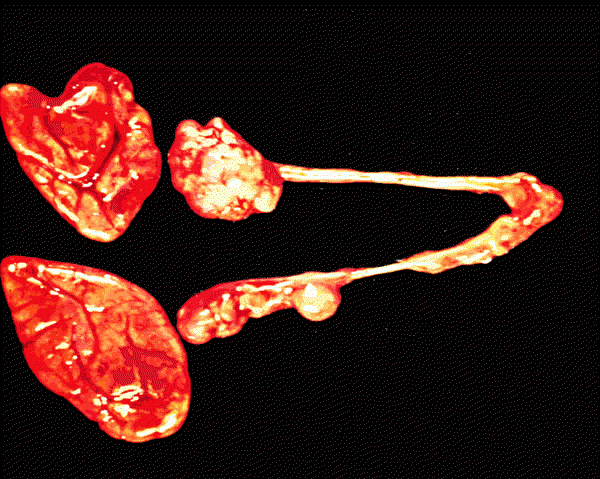
"Hyperplastic adrenals and poorly developed ovaries, uterine tubes and uterus in a female child with congenital adrenal hyperplasia"; 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center;For more information concerning endocrine pathology
Congenital adrenal hyperplasia
Caused by cortisol synthetic defects
autosomal recessive mutations
Most common adrenal disorder of childhood and infancy
Late-onset adrenal hyperplasia cause:
5%-25% of hirsuitism and oligomenorrhea cases in women
Pregnancy at risk for congenital adrenal hyperplasia:
dexamethasone administration to the mother protects
decrease or lack of cytochrome P450c21 (21ß hydroxylase) activity (95% frequency) which results in:
cortisol synthesis reduction
compensatory increase of ACTH release
Other deficiencies:
P450c18, P450c17a , P450c11ß, 3ß- hydroxysteroid dehydrogenase
Increased compensatory ACTH can result in normal cortisol levels if sufficient P450c21 activity is present; however the gland will:
become hyperplastic
produce excessive precursors such as 17-hydroxyprogesterone -- diverted to androgen pathways-- leading to virilization.
Diagnosis:
Excessive 17-hydroxyprogesterone is metabolized in the liver to pregnanetriol, detected in large amounts in the urine.
Most reliable detection: increased plasma 17-hydroxyprogesterone to ACTH stimulation
Female:
adrenal virilization associated with:
ambiguous external genitalia (female pseudohermaphroditism)
enlargement of the clitoris
partial/complete labial fusion
urogenital sinus (possible)
Male: enlarged genitalia
Postnatal period:
female virilization
isosexual prococity in the male
excessive androgen levels:
accelerated growth
early epiphyseal closure; growth stops --truncal development continues-- (short child with well-developed trunk.
excessive desoxycorticosterone production: hypertension
adrenal and gonadal defects increased 11-desoxycorticosterone levels-- mineralocorticoid excess signs/symptoms:
hypertension
hypokalemia
Infant with congenital adrenal hyperplasia:Presenting Symptoms --
Treatment
If acute adrenal crisis:
IV cortisol
mineralocorticoid
electrolyte solutions
After stabilization:
hydrocortisone (Cortef, Solu-Cortef)-- adjusted as required
alternative: prednisone (Deltasone)
mineralocorticoids may be required (fludrocortisone (Florinef))
bilateral adrenal hyperplasia due to pituitary adenoma (basophilic tumor)
about 20 to 25 percent of Cushing's syndrome patients have adrenal neoplasm
ACTH Hypersecretion
Other Causes:
adrenal tumors
nodular adrenal hyperplasia-- causes:
familial autoimmune (children/young adults)-- pigmented multinodular cortical dysplasia
hypersensitivity to gastric inhibitory peptide (increased peptide receptor expression in adrenal cortex)
ectopic, nonendocrine tumors that produce ACTH
three times greater in women
most frequent age of onset: 30s to 40s
Abnormal fat deposition:
truncal obesity-- mesenteric bed
rounded face ("moon facies")
interscapular area ("buffalo hump")
hirsuitism;
effects of protein loss --
muscle wasting
skin thinning
striae-- secondary to weakening/rupture of dermal collagen fibers
poor wound healing
bruising
osteoporosis
mental disorders--
irritability/emotional lability
severe depression, confusion, psychosis
diabetes
frequency < 20%
impaired glucose tolerance:
increased hepatic gluconeogenesis
insulin resistance
hypertension-- common

"Abdominal striae in a patient with trucal obesity in Cushing syndrome"; Ó 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center. For more information concerning endocrine pathology
Surgical pituitary tumor removal
Irradiation/removal of pituitary adenomas (ACTH producing)
"Autopsy case. Unoperated macroadenoma of pituitary."
Ó 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center.
For more information concerning endocrine pathology
microadenoma surgical resection is associated with recurrence rate that may be greater than 20 percent
About 20% -- 25% of Cushing's patients have an adrenal neoplasm.
usually unilateral; about 50%, malignant
Adrenal carcinoma diagnosis: approximate three-year post-diagnosis survival time
associated with liver and lung metastasis
primary chemotherapeutic agent: mitotane which suppresses cortisol production, decreasing plasma and urine steroid levels
Mitotane: -- relatively selective for glucocorticoid-secreting adrenal cortex zone; extra-adrenal cortisol metabolism may also be affected
one-third of patients: tumor and metastasis regress
in many patients:
steroidogenesis -- inhibited
no regression of tumor metastases
resection of hyperplastic adrenals -- if source of ACTH is apparent
cure rate approximates 100%
adverse effects:
lifelong glucocorticoid and mineralocorticoid replacement
10% to 20% probability of developing pituitary tumor over following ten year period (Nelson's syndrome)
in surgical approach not feasible, "medical" adrenalectomy may be required:
"Medical" adrenalectomy:
high-dose ketoconazole (Nizoral) (inhibit steroidogenesis)
mitotane (Lysodren) and/or aminoglutethimide (blocks steroid synthesis) and metyrapone
Patient management:(surgical intevention)
Before surgery: large doses of cortisol
During and immediately after surgery: large doses of cortisol
Tapered cortisol to normal replacement doses (long-term maintenance)
Diagnosis: Dexamethasone suppression testing
Initial Screening
demonstration of increased cortisol production (8 a.m. plasma cortisol > 140 nmol/L (5 microgram/dL);
cortisol > , following 1 mg dexamethasone (Decadron) at midnight
Definitive diagnosis:
failure of urinary cortisol to fall to less than 80 nmol/d (30 microgram/d) or
plasma cortisol to fall to less than 140 nmol/L (5 microgram/dL) following standard low-dose dexamethasone (Decadron) suppression testing (0.5 mg every six hours for 48 hours)
twice as common in women as in man
most often presents between 30 in 50 years of age
adrenal adenoma -- excessive aldosterone production
unilateral adenoma (usually small; either side)
Conn's syndrome

Conn's --primary aldosteronism:"Adrenal cortical adenoma There is a solitary, well-demarcated mass with the typical mottled yellow color of adrenal cortex."Ó 1999 KUMC Pathology and the University of Kansas, used with permission; courtesy of Dr. James Fishback, Department of Pathology, University of Kansas Medical Center.For more information concerning endocrine pathology
hyperplastic adrenal glands -- abnormal secretion
malignant tumor
adrenal carcinoma (rare)
Physiological Effects of aldosterone hypersecretion:
increased renal distal tubule or exchange of sodium for secreted potassium and hydrogen ions -- body potassium depletion/hypokalemia
Diagnosis--Criteria:
diastolic hypertension (no edema)
renin hyposecretion (low plasma renin activity)
renin secretion does not increase with volume depletion
aldosterone hypersecretion that is not suppressed with volume expansion
diastolic hypertension (not very severe)
secondary to increase sodium reabsorption/volume expansion
headaches
polyuria, polydipsia
impairment of urinary concentrating ability
weakness
due to effects of potassium depletion
tetany
Electrocardiographic changes -- consistent with potassium depletion (hypokalemia-- which increases ectopy)
prominent U waves
cardiac arrhythmias
premature contractions
Many effects secondary to potassium loss associated with:
hypokalemia
may be severe (< 3 mmol/L)
hypernatremia-- due to:
sodium retention
water loss from polyuria
metabolic alkalosis-- due to
urinary hydrogen ion loss
movement of hydrogen ion into potassium-depleted cells
alkalosis enhanced by potassium deficiency which increases proximal convoluted tubule capacity to reabsorb filtered bicarbonate.
Due to adenoma -- usually treated surgically
may be treated by:
sodium intake restriction
aldosterone antagonist (spironolactone (Aldactone))
prolonged medical management (chronic therapy) may be side effect limited (males)
gynecomastia
decreased libido
impotence
Due to idiopathic bilateral hyperplasia
symptomatic hypokalemia treated by:
spironolactone (Aldactone)
triamterene (Dyrenium)
amiloride (Midamor)
surgery if pharmacological treatment fails
Cause: appropriate increased aldosterone production due to renin-angiotensin system activation
accelerated hypertension
presence of edema disorder
normal physiologic response to estrogen-induced increased plasma renin substrate and plasma renin activity and to antialdosterone actions of progestogens
in hypertension -- cause:
over production of renin (primary reninism)
renin over production secondary to reduced renal blood flow/perfusion pressure
reduced renal artery lumen secondary to atherosclerosis or fiber muscular hyperplasia
severe arteriolar nephrosclerosis (malignant hypertension)
profound renal vasoconstriction (accelerated hypertension)
may be caused by rare renin-producing tumors (primary reninism)
juxtaglomerular cell tumor
Physiological characteristics:
increased plasma renin activity
moderate/marked increases in aldosterone levels
hypokalemic alkalosis
Adrenocorticosteroids and Fetal Lung Maturation
Lung maturation is dependent on fetal cortisol
If delivery is expected before 34 weeks gestation, maternal glucocorticoid supplementation reduces likelihood of respiratory distress syndrome.
Betamethasone (Celestone) preferred due to reduced protein binding -- making more available for placental transfer to the fetal circulation.
|
Disorder |
Some Examples |
|
Allergic reactions |
angioneurotic edema, asthma, contact dermatitis, drug reactions, allergic rhinitis, urticaria |
|
Collagen-vascular pathology |
giant cell arteritis, lupus erythematosus, polymyositis, rheumatoid arthritis, temporal arteritis |
|
Eye diseases |
allergic conjunctivitis, optic neuritis |
|
Gastrointestinal |
inflammatory bowel disease than |
|
Hematologic |
acute allergic purpura, leukemia, autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, multiple myeloma |
|
Infections |
gram-negative septicemia and |
|
Inflammatory disorders of joints/bones |
arthritis, bursitis,tenosynovitis |
|
Neurologic |
cerebral edema, multiple sclerosis |
|
Organ Transplantation |
prevention/treatment of rejection (immunosuppression) |
|
Pulmonary |
bronchial asthma, prevention of infant respiratory distress,sarcoidosis, aspiration pneumonia |
|
Renal |
nephrotic syndrome |
|
Skin |
atopic dermatitis, dermatoses, mycoses fungoides, seborrheic dermatitis |
|
Thyroid |
malignant exophthalmos, subacute thyroiditis |
|
adapted from Table 39-2; Goldfien, A.,Adrenocorticosteroids and Adrenocortical Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, p 643. |
moon facies
fat redistribution; e.g. truncal obesity
acne
hirsuitism
insomnia, increased appetite
weight gain
muscle wasting
skin thinning, bruising
hyperglycemia
osteoporosis, diabetes, aseptic hip necrosis
wound healing
peptic ulcer development
myopathy (triamcinolone)
nausea, dizziness, weight loss (triamcinolone, methylprednisolone)
psychosis (large dose corticosteroids)
subcapsular cataracts
increased intraocular pressure/glaucoma
benign intracranial hypertension
growth retardation and children
cortisone/hydrocortisone in greater than physiologic amounts: mineralocorticoid effects:
sodium/fluid retention
potassium loss-- hypokalemia
hypochloremic alkalosis
hypertension
significant adrenal suppression observed with extended treatment
patient should receive supplemental steroid in cases of accidental trauma/surgery
the presence of adrenal suppression requires slow tapering of adrenocorticoid dosage
patient should be observed to detect development of:
hyperglycemia
glycosuria
Na retention with edema
hypertension
hypokalemia
peptic ulcer
osteoporosis
hidden infections
peptic ulcer disease
heart disease/hypertension with congestive heart failure
psychoses
diabetes
osteoporosis
glaucoma
herpes simplex infection
probably not appropriate as a therapeutic agent unless androgen increase is desired
ophthalmic -- eye disease
intra-articular -- joint disease
hydrocortisone enemas-- ulcertive colitis
aerosols (e.g.beclomethasone) -- asthma
nasal spray (beclomethasone, triamcinolone, flunisolide) -- allergic rhinitis
ointments, creams -- dermatological applications
Mineralocorticoids (Aldosterone, Desoxycorticosterone, Fludrocortisone)
Most important mineralocorticoid: aldosterone
secondarily: desoxycorticosterone (DOC)
Most commonly prescribed salt-retaining hormone: fludrocortisone
synthesized in zona glomerulosa of the adrenal cortex
regulation -- ACTH, angiotensin
promotes sodium reabsorption by distal renal tubule (loosely coupled to potassium and hydrogen on and secretion)
Excessive aldosterone-- (secondary to tumor/overdosage):
hypernatremia
hypokalemia
metabolic alkalosis
hypertension
increased plasma volume
Mechanism of Action:
mineralocorticoid binding to cytoplasmic receptor (e.g.renal collecting tubule principal cells)
subsequent steps similar to those described for glucocorticoids
precursor to aldosterone
secretion of DOC controlled by ACTH
DOC secretion enhanced in abnormal conditions e.g.:
adrenal carcinoma
congenital adrenal hyperplasia (with reduced P450c11or P450c17)
Fludrocortisone (Florinef)
most widely used mineralocorticoid
glucocorticoid and mineralocorticoid activity
used in management of adrenocortical insufficiency
Large amounts of dehydroepiandrosterone (DHEA) secreted; smaller amounts of androstenedione and testosterone secreted
probably contribute to normal maturation processes
Antagonists of Adrenocortical Agents
Synthesis Inhibitors and Glucocorticoid Antagonists:
selective inhibitor of steroid synthesis (inhibiting 11-hydroxylation which interferes with cortisol and corticosterone synthesis)
produces dizziness and gastrointestinal disturbance
not widely used for Cushing's syndrome
reduces cortisol production to normal in some patients with:
adrenal tumor
ectopic ACTH syndromes
hyperplasia
may be useful in management of severe effects of cortisol excess on a temporary basis
Major adverse effects:
salt and water retention
hirsuitism
most commonly used in adrenal function tests
blocks cholesterol to pregnenolone conversion
reduces synthesis of all hormonally active steroids
used together with dexamethasone (Decadron) or hydrocortisone (Cortef, Solu-Cortef) to eliminate estrogen and androgen production in patients with breast carcinoma
used with ketoconazole (Nizoral) to reduce steroid secretion impatience with Cushing's syndrome (due to adrenocortical cancer -- not responding to mitotane)
Ketoconazole: (Nizoral)
antifungal imidazole derivative: potent, nonselective adrenal and gonadal steroid synthesis inhibitor
inhibition of P450 enzymes induces a compensatory increase in ACTH production and increases in progesterone, aldosterone and suppression of plasma renin activity
occasionally causes gynecomastia by increasing estradiol/testosterone plasma ratio
use for treating patients with Cushing's disease
synthetic, partial agonist steroid
binds to glucocorticoid and progesterone receptors
treatment of Cushing's syndrome (experimental)
Mitotane-- toxicities resulted in drug withdrawal from U.S. market
interferes with biosynthetic pathways in human adrenal cortex
Trilostane-- similar to aminoglutethimide;3ß-17 hydroxysteroid dehydrogenase inhibitor
Mineralocorticoid Antagonists:
Spironolactone: (Aldactone)
used in treating primary aldosteronism
diagnostic use
management of symptoms while patient awaits adenoma surgical removal
reating hirsuitism in women
Adverse effects:
hyperkalemia
menstrual abnormalities
gynecomastia
sedation
headache
gastrointestinal disturbances
skin rashes
Drospirenone-- progestin; antagonizes aldosterone effects
Goldfien, A.,Adrenocorticosteroids and Adrenocortical Antagonists, in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, pp 635-650.
Williams, G. H and Dluhy, R. G. , Diseases of the Adrenal Cortex, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, pp 2035-2056