|
|
|
Anesthesia Pharmacology Chapter 15: Cardiac Anesthesiology
Cardiac Effects: Inhalational Agents
1Isoflurane and other agents: Effects on myocardial inotropic state
Isoflurane appears to be less of myocardial depressant compared to halothane or enflurane.
Reduction in contractility depression with isoflurane compared to these agents has been noted in both human and animal studies. In a dog model using preload-recruitable stroke work slope (PRSW slope) as an index of contractility, isoflurane and desflurane produced similar contractility depression.
Since myocardial contractility could be influenced by the autonomic reflex state, desflurane and isoflurane effects in myocardial contractility were compared in the presence of autonomic blockade. In that case, again, similar myocardial depressant effects were observed, suggesting that potentially differential autonomic effects induced by these agents was not an important factor in causing myocardial depression. Extending this analysis to sevoflurane, a comparable level of myocardial negative intropism was found for that agent compared to desflurane and isoflurane.
In the sevoflurane study, PRSW was used as the index of contractility and finding agreed with that obtained another study which used load-dependent maximal pressure development rate (dP/dtmax) as the measure contractility. Using echocardiography to assess inhalational agent effects on contractility, enflurane and sevoflurane effects were compared in humans. The slope of the left ventricular and-systolic wall stress (LVESWS) -velocity of circumferential fiber shortening, heart rate corrected (Vcfc) aka [LVESWS-Vcfc] relationship was used as the load-independent contractile state index. In this system sevoflurane administration did not depress myocardial contractility as much as that observed with enflurane.
![]() 1Overall,
the efficacy of volatile inhalational agents in depressing myocardial
contractility would be: enflurane = halothane > isoflurane =
desflurane = sevoflurane.
1Overall,
the efficacy of volatile inhalational agents in depressing myocardial
contractility would be: enflurane = halothane > isoflurane =
desflurane = sevoflurane.
|
|
= |
|
> |
|
= |
|
= |
|
1Inhalational Agent Effects in the Abnormal Myocardium
1A generalization concerning the volatile agent effects on myocardial contractility would be that contractility in the abnormal heart appears more sensitive to influences by a particular anesthetic concentration, compared to the normal heart.
Papillary muscle preparations in a feline CHF model allowed analysis of enflurane and fluorxene effects on maximal muscle shortening velocity (Vmax), maximal force development (Fmax)) and maximal rate of force development (dF/dtmax)). In this study it was not possible to demonstrate a difference between the CHF model and the normal heart.
In a different study, but using the same model, isoflurane did cause greater contractility depression in the CHF preparation compared to the normal preparation. Perhaps this type of an effect might explain the possibly narrower range of anesthetic concentration is that may be tolerated by the CHF patients
|
|
1,7Congestive heart failure animals dotted line; normal animals, solid line. Results indicated increased, dose-dependent isoflurane induced Vmax depression
To study effects of some anesthetics in a myocardial infarction model, myocardial infarction was induced by ligation of three left ventricular branches of the left anterior descending coronary artery in dogs.
Halothane and nitrous oxide following thiopental induction were the agents studied 7-10 days after the ligation procedure had been performed. In this system no change in heart rate (HR), blood pressure (BP), SVR, or left ventricular dP/dt was noted.
The following parameters decreased: stroke volume (SV), cardiac output, and aortic blood flow acceleration.
The following parameters increased: left ventricular end-diastolic pressure (LVEDP). These changes were assessed after 90 minutes under anesthesia.
Generally these findings were similar to findings reported another study is in which halothane was evaluated in the normal animal. Authors utilizing the myocardial infarction model suggested that halothane appeared to be well-tolerated.
Furthermore if the region of ischemia appears to be less than 25% of the left ventricular myocardium, generalized hemodynamic parameters may not change following halothane administration, even in the presence of significant of localized severe dysfunction.
Most of the evidence concerning nitrous oxide effects on myocardial mechanics suggest that although nitrous oxide is a myocardial depressant, it is less so compared to the potent volatile agents. Furthermore, the negative inotropic effect is not secondary to hypoxia. One reason why it is been difficult to resolve this issue conclusively is that nitrous oxide increases sympathetic nervous system activity, which may act as a counter to the agent' s depressant effects. The myocardial depressant effect appears to be a consistently observed effect in humans with heart disease.
8In an animal study in which nitrous oxide effects were analyzed in a chronically instrumented dog, ntirous oxide was administered to animals had been anesthetized with either isoflurane or sufentanil. In both the isoflurane and sufentanyl groups, there was a dose-dependent contractility depression noted with nitrous oxide. Probably nitrous oxide is a weak direct myocardial depressant, an effect which may be offset by sympathetic activation.
Assessment of Diastolic Function
The significance of diastolic function is in recognition of the fact that many individuals with heart failure (perhaps as many as 40%) abnormal systolic function. Accordingly, it is of interest to know the influence of inhalational agents on diastolic function.
Despite being thought of as a relaxation phase in the cardiac cycle, diastole is an energy-requiring an activity since calcium must be transported back to sarcoplasmic reticulum storage sites. The diastole is often divided into four phases: isovolumic relaxation, early rapid filling, diastasis, and finally atrial kick which could be the atrial contribution to ventricular filling at the diastole.
The significance of end-diastolic volumes is reflected in the Frank Starling relationship.
In more molecular terms and fiber length-force relationships focus on sarcomere length.
Maximal force is developed when initial,
resting sarcomere length is about 2-2.4 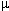 m.
m.
With this dimension, there is optimal overlapof thin and thick filaments corresponding to maximal numbers of cross bridge attachments.
Myocardial stretch with increased load appears to increase the monofilament sensitivity to Ca2+ with resulting increased force of attraction secondary to increases in the affinity of troponin C for Ca2+ . As sarcomere length is increased beyond a certain point, cardiac muscle force development declines as a result of reduced filament overlap and less cross bridge cycling. By contrast, a sarcomere length shorter than the optimal distance, thin filaments that extend from adjacent Z lines overlap each other in the sarcomere central region -- a condition which also will reduce contractile force.
|
1Assessment of Diastolic State
In order to assess effects of inhalational agents on diastolic function, it is necessary to identify means of assessing diastolic function.
The following approaches are available:
quantify isovolumic relaxation
quantify diastolic filling (early)
quantify chamber stiffness
and quantify myocardial stiffness.
Isovolumic relaxation is represented as a peak
rate of intraventricular pressure decrease (-dP/dtmin) or as
a time constant of pressure decline (tau,  ).
A time constant is the length of time required for a decline of about
63% of the initial value.
).
A time constant is the length of time required for a decline of about
63% of the initial value.
As  increases, the rate of ventricular isovolumic relaxation decreases.
increases, the rate of ventricular isovolumic relaxation decreases.  is influenced by ventricular loading characteristics and heart rate
but reflects changes in myocardial relaxation rate.
is influenced by ventricular loading characteristics and heart rate
but reflects changes in myocardial relaxation rate.
 increases and ventricular relaxation is slowed by left ventricular
hypertrophy, dilated cardiomyopathy, negative inotropic agents, and
ischemia
increases and ventricular relaxation is slowed by left ventricular
hypertrophy, dilated cardiomyopathy, negative inotropic agents, and
ischemia
 is decreased by catecholamines and sympathetic stimulation,
meaning that it takes less time to drop to 63% of the initial value.
i.e. faster relaxation.
is decreased by catecholamines and sympathetic stimulation,
meaning that it takes less time to drop to 63% of the initial value.
i.e. faster relaxation.
Early diastolic filling is estimated using peak rate of ventricular filling, an invasive measurement, as the rate of change of ventricular volume (dV/dt) , or as the rate of change of segment length dL/dt.
Chamber stiffness, (dP/dV) which is the inverse of compliance (dV/dP) , is described by a slope Kp, of the dP/dV-ventricular pressure relationship. This slope is sensitive to left ventricular volume and mass, thus limiting its specificity.
Myocardial stiffness is a characteristic of the myocardium without effects of wall thickness, ventricular geometry or pericardial pressure and can be estimated using a ventricular stress-strain relationship, a difficult and complex approach with limited utility.
1Diastolic function is most commonly assessed noninvasively using Doppler-echocardiography.
Flow data is obtained at either the pulmonary veins or mitral valve.
Doppler echocardiographic displays involve time on the x-axis and flow velocity on the y-axis.
Typically, transmitral blood flow is biphasic with an early rapid filling component (E-wave) and a later component, diastole atrial filling (A-wave). Transaortic flow is also detected in this acquisition because of the spatial proximity of the aortic valve.
The isovolumic relaxation time is the time between aortic valve closure and mitral valve opening. Diastasis, the diastolic third phase, is represented by the limited flow between the E- and A- waves.
1,9Left ventricular inflow velocity
IVRT: isovolumic relaxation time (time from aortic valve closure to onset of mitral flow)
AVD: aortic valve closure
E: larger early (E) diastolic filling velocity
A: smaller atrial filling velocity
DT: Deceleration time from peak velocity to decline to the baseline
1Doppler assessment of left ventricular diastolic filling includes the following parameters: E velocity, A velocity, E/A ratio as well as time-velocity and integrals (TVI) IVRT, time to peak E velocity , and measures of E-wave acceleration and deceleration also are useful in describing diastolic ventricular function.
Diastolic function abnormalities reflected in transmitral flow are classified as either (a) impaired ventricular relaxation or (b) decreased diastolic compliance.
Relaxation abnormalities reflected in IVRT prolongation with E-wave reduction velocity and A-wave increased velocity.
Reduced ventricular compliance is associated with a steeper E-wave deceleration slope with increased E-wave velocity and decreased (a) time to peak E-wave velocity, (b) peak A-wave velocity and (c) IVRT.
This latter pattern of transmitral flow characteristics is an index of advanced heart disease and is characterized by increased filling pressures, atrial systolic failure, and a reduction of functional capacity.
A negative aspects of transmitral flow as an indicator of diastolic dysfunction is a lack of specificity.
Alterations in diastolic function indicated by this method can be affected by:
heart rate
respiration
preload
age
resence of mitral valve anatomical dysfunction
left ventricular dysfunction and
atrial ventricular interval duration . As an example, increasing preload significantly increasesE-wave velocity. Also, should a patient have an abnormality of ventricular relaxation characterized by small E-wave, large A-wave) the presence of increase left atrial pressure will result in a normal E/A ratio with a "pseudonormalized" transmitral filling pattern. Examination of pulmonary venous flow patterns in a assistant analysis
There is a progressive decline in E velocity transmitral flow rates are measured more distally (apically) from the mitral valve in those patients with diastolic dysfunction and pseudonormal transmitral flow patterns.
1Pulmonary venous flow is triphasic and nature-the three components include: a systolic component, a diastolic component, and a flow reversal and end-diastole associated with atrial contraction.
1,9Pulmonary venous flow velocity
MVC: mitral valve closure
MVO: mitral valve opening
AR: pulmonary venous peak atrial reversal flow velocity
D: pulmonary venous diastolic flow velocity
1,9Corresponding pulmonary venous flow velocity with transmitral flow velocity
1Pulmonary venous flow analysis measurements include velocity and/or time-velocity integrals (TVI) of the diastolic, systolic, and atrial elements as well as ratio of these parameters to total pulmonary venous flow.
With impaired ventricular relaxation, the diastolic component is reduced with accentuation of atrial reverse flow. Abnormal ventricular compliance shows significant diastolic blood influx with prominent atrial flow reversal.
1Pseudonormalized patterns with abnormal relaxation compared to normal transmitral flow pattern with normal diastolic function can be differentiated by pulmonary venous flow analysis.
Pulmonary venous flow pattern analysis exhibits limited specificity and can be affected by not only changes in ventricular diastolic performance but also by other factors that influence transmitral flow patterns (noted above).
Pulmonary venous flow analysis has been used clinically to identify individuals with increased left atrial pressure which is considered a late marker of diastolic dysfunction.
1,10Systolic component of pulmonary venous flow is > 55% of total pulmonary venous flow, predicts with both high specificity and sensitivity left atrial pressure < 15 mm Hg. 1,11Systolic pulmonary venous flow component < 40% of total pulmonary venous flow predicts diastolic filling pressures > 18 mm Hg.
![]()
1Park, KW, Haering, JM, Reiz, S, Lowenstein, E Effects of Inhalation Anesthetics on Systemic Hemodynamics and the Coronary Circulation in Cardiac Anesthsia, Fourth Edition (Kaplan, JA, ed; Reich, Dl and Konstadt, SN, Assoc eds) W.B. Saunders Co. A Division of Harcourt Brace and Company, Philadelphia, 1999. This chapter is the primary reference for all above material, except as noted.
2Suga, H, Sagawa, K, Shoukas, AA: Load independence of instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res 32: 314, 1973. -second sourced from reference 1.
5Van Trigt, P, Christian, CC, Fagraeus, L, et al: "Myocardial depression by anesthetic agents [halothane, enflurane, and nitrous oxide]: Quantitation based on end-systolic pressure-dimension relations" Am J Cardiol 53: 243, 1984---second sourced from reference 1.
7Kemmotsu, O, Hashimota, Y, Shimosato, S: Inotropic effects of isoflurane on mechanism of contraction in isolated cat papillary muscles from normal and failing hearts. Anesthesiology 39: 470, 1973 --second sourced from reference 1.
8Pagel, PS, Kampine, JP, Schmeling, WT, Warltier, DC: Effects of nitrous oxide on myocardial contractility as evaluated by the preload recruitable stroke work relationship in chronically instrumented dogs. Anesthesiology 73: 1148, 1990. --second sourced from reference 1.
9Klein, AL, Taijik, AJ: Doppler assessment of pulmonary venous flow in healthy subjects and in patients with heart disease. J Am Soc Echocardiogr 4: 379-392, 1991--second sourced from reference 1.
10Kuecherer, HE, Muhiudeen IA, Kusumoto FM et al: Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow. Circulation 82: 127, 1990.--second sourced from reference 1.
11Rossvoll, O, Hatle, LK: Pulmonary venous flow velocity recorded by transthoracic Doppler ultrasound: Relation to left ventricular diastolic pressures JACC 21: 1687, 1993.--second sourced from reference 1.