Anesthesia Pharmacology Chapter 16: Pulmonary Anesthesiology
|
|
|
The Effects of Thoracic Epidural Analgesia with Bupivacaine 0.25% on Ventilatory Mechanics in Patients with Severe Chronic Obstructive Pulmonary Disease
1Gruber, EM, Tschernko, EM, Kritzinger, M, Deviatko, E, Wisser, W, Zurakowski, D, and Haider, W, The Effects of Thoracic Epidural Analgesia with Bupivacaine 0.25% on Ventilatory Mechanics in Patients with Severe Chronic Obstructive Pulmonary Disease Anesth Analg 2001; 92: 1015-1019.
1Overview:
This article considers the following problem: on one hand thoracic epidural anesthesia (TEA) is effective in management of post-thoracic surgical pain; such management may be especially important in reducing pain-related respiratory complications in patients who have pulmonary dysfunction.
As noted in an earlier chapter, however, TEA while providing effective pain management, also may paralyze certain respiratory muscles and induce unfavorable bronchomotor tone changes.
The research in this article evaluates the effect of bupivacaine 0.25% used in thoracic epidural anesthesia in those patients with severe COPD.
1Summary:
This research analyzed effects of TEA on the following parameters: maximal inspiratory pressure, breathing pattern, ventilatory mechanics and gas exchange.
The patient group consisted of 12 end-stage COPD patients.
Following TEA, minute ventilation, due to increased tidal volume was noted. Moreover, these changes were associated with an increased in peak inspiratory flow rate with a decrease in pulmonary resistance. The following parameters appeared unchanged by TEA: peak inspiratory flow rate, dynamic intrinsic positive end-inspiratory pressure, breathing work, PaO2 and maximal inspiratory pressure.
The authors conclude that these results indicate that TEA using 0.25% bupivacaine can be used safely in end-stage COPD patients. The clinical implication was that TEA using the particular local anesthetic at the specified dosage would not worsen ventilatory mechanics or reduce inspiratory muscle strength in this patient group and therefore this protocol may be used safely in patients with end-stage COPD.
The specific problem addressed in this research is introduced of course in the "Introduction" part of the paper.
There must be some outstanding question to which this research is addressed.
The general idea, as you know, begins with the recognition that thoracotomy produces significant post-operative pain and that this post-operative pain causes impairment of ventilatory mechanics and gas exchange.
Not surprisingly, there would be a subset of patients very sensitive to even slight impairment of pulmonary function. An example of such a group of patients would be the COPD group.
A number of factors may degrade perioperative pulmonary function and oxygenation.
These factors include ventilation-perfusion mismatching, shunt, atelectasis, and that these factors could be affected adversely by immobilization, continued presence of anesthetic drug effects and pharmacological effects of analgesic drugs and sedative agents.
Postoperative impairment of ventilation may be reduced by TEA and consequently there may be a reduction postoperative pulmonary-related complications.
The problem is conflicting research results on this point. On one hand, a number reports suggest that use of TEA may reduce adverse perioperative pulmonary changes.
By contrast, other studies indicate no change in the likelihood of postoperative pulmonary complications when a comparison is made between postoperative epidural analgesic with systemic opioid administration.
In the latter case, patients studied at normal preoperative lung function.
TEA may induce a reduction in ventilatory reserve, peak negative and positive airflow, inspiratory capacity, vital capacity, and expiratory reserve volume. Furthermore, COPD patients, at least severe COPD patients, utilize the abdominal and intercostal muscles to ensure adequate flow.
Therefore, motor blockade of these muscles following TEA could decrease peak inspiratory and expiratory flow rates (PIFR, PEFR respectively) and reduced flow rates could limit ventilatory reserve leading to inadequate spontaneous ventilatory activity in end-stage emphysema individuals.
Also, potential motor blockade could reduce cough.
Thoracotomy itself, because of respiratory muscle disruption, postoperative pain, and visceral stimulation causing reduced motoneuron output, causes an impairment of ventilatory mechanics, respiratory muscle function, and breathing pattern.
TEA would be effective in management of postoperative pain but could have adverse consequences in terms of postoperative spontaneous breathing and, because of suppression of cough, inhibit secretion removal.
In patients with normal lung function and in patients with fairly mild COPD, TEA did not appear to adversely influence ventilatory mechanics.
But the authors recognize that TEA effects on ventilatory mechanics could be quite different in patients with end-stage COPD, providing the rationale for this study to assess TEA effects (bupivacaine 0.25%) on breathing pattern, gas exchange, maximal inspiratory pressure (MIP) and ventilatory mechanics in the patient group characterized by severe COPD.
|
|
|
The theoretical effects of TEA on ventilatory mechanics noted above may provide a rationale for the study but again the question is not whether or not TEA effects might occur but only whether they in fact do occur. Research addresses the question of whether or not the phenomenon in fact occurs, either proving or disproving some hypothesis. But whether experiment (experience) allows acceptance or rejection of the hypothesis critically depends on the quality of the experimental approach. One of the difficulties in evaluating a specific research study has to do with the familiarity of the reader with the specific experimental challenges. If the authors address experimental limitations, the ability of the reader to assess the strength of the conclusions is enhanced.
1Methods:
The strength of the conclusions of any study is related closely to the methods employed in acquiring data. In this study, 12 patients were evaluated. Six women and six men were included, each characterized as ASA III, all with severe COPD which, defined as FEV1 < 40%. Patients were scheduled for lung volume reduction surgery. Monitoring included ECG lead II and pulse oximetry. Fluid administration was provided through a catheter in a peripheral vein. Invasive blood pressure monitoring and blood gas analysis utilized the radial artery. Following administration of lactated Ringer's solution [10 ml/kg], epidural catheter insertion occurred at the T 4-5 level with position verification using 4ml 2% lidocaine injection (1:200,000 epinephrine).
Ventilatory mechanics baseline was determined prior to epidural catheter insertion. Nasopharynx local anesthesia was provided using lidocaine spray and bronchospasm avoided by aerosolized salbutamol administration (3 puffs). Ventilatory mechanics were measured utilizing an esophageal balloon catheter inserted through the nose into the esophagus. Baseline ventilatory measurements followed a 15 minute rest period and measurements also included arterial blood gases.
Airflow was measured utilizing a flow sensor (Bicore CP-100) which was connected to a tightly fitted facemask. Assessment of fit was based on comparing inspiratory and expiratory volumes. Adequacy of fit was defined as < 5% difference between these volumes. Airflow was restricted to nasal flow (mouth closed)
CP-100: This pulmonary monitor has been described as using microprocessor technology and utilizes an esophageal balloon catheter to measure airway pressure and airway flow. Esophageal pressure (Pes) is an indirect measure of intrapleural pressure and provides an indication of breathing work. This type of monitor also calculates other indices of ventilatory mechanics, patient-ventilator synchrony, and respiratory strength. Use of the CP-100 unit has been validated in previous studies2,3
Tidal volume was obtained by integration of the flow signal. Airway pressure (Paw) and measurements were obtained through catheter attached to the flow sensor and the esophageal pressure assessed by a 7 FR nasogastric tube which incorporated the esophageal balloon. An occlusion test verified correct positioning of the balloon catheter. The esophageal balloon and flow sensor was attached to the monitor which provided a real-time display of airflow, volume (V), Paw and Pes data. Minute ventilation (VE) and breathing pattern, defined as tidal volume (VT), respiratory frequency (Rf), inspiration duration (TI), expiration duration (TE) and the duty cycle (Ti/Ttot), were determined from the flow signal.
Total resistive breathing work (WOB) was provided by the monitoring device which calculated the area under the PES vs. lung volume curve. WOB was determined for each breath. Pulmonary resistance (RL) was also calculated by the monitor and was defined as the difference in transpulmonary pressure / the difference in flow taken at the same volume during both inspiration and expiration.
Dynamic intrinsic positive and-expiratory pressure (PEEP
i,dyn) was measured and considered equal to the absolute
change in Pes from the inspiratory effort onset
to the onset of inspiratory airflow. Pes
tracings were evaluated before and after TEA in order to detect
expiratory muscle activation that could influence measurement results.
The maximal Pes change during an entire respiratory
cycle 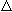 Pes
was evaluated for 39 breaths before and after TEA with measurements
obtained in patients quietly breathing room air and a 30 ° head-up
position. During these resting circumstances, WOB, PEEP i,dyn
and RL was calculated from the 39 breaths; however,
some measurements were excluded if these measurements differed from the
mean by greater than 2 SD. Extreme values were considered artifactual
and were probably caused by coughing or swallowing. During assessment of
ventilatory mechanics, arterial blood gas data was obtained.
Pes
was evaluated for 39 breaths before and after TEA with measurements
obtained in patients quietly breathing room air and a 30 ° head-up
position. During these resting circumstances, WOB, PEEP i,dyn
and RL was calculated from the 39 breaths; however,
some measurements were excluded if these measurements differed from the
mean by greater than 2 SD. Extreme values were considered artifactual
and were probably caused by coughing or swallowing. During assessment of
ventilatory mechanics, arterial blood gas data was obtained.
Following the baseline measurements described above of the ventilatory mechanics, MIP measurement by flow sensor during a maximal inspiratory effort (Mueller maneuver i.e. inspiring against a closed glottis) against the occluded inspiratory limb was measured with the highest value from three consecutive maneuvers used in subsequent analysis.
Patients rested two minutes between these maneuvers.
Following baseline measurements and catheter position confirmation, lidocaine 2% + epinephrine (1: 200,000) and 10-12 ml bupivacaine 0.25% was administered by epidural route. Anesthesia spread was determined by pinprick test and warm/cold discrimination 45 minutes following bupivacaine injection. If spread of anesthesia was appropriate, defined as a minimum extension of blockade from T2-T8, arterial blood gas sampling was performed with ventilatory mechanics measured as noted above.
|
|
Continuous data was presented as the mean +/- standard deviation (SD). Continuous outcome variables did not show a significant departure from a normal distribution (Kolmogorov-Smirnov goodness-of-fit test).
Paired t-tests were used to determine mean differences pre-and post-TEA.
Repeated-measures analysis of variance determined if changes in ventilatory mechanics or breathing patterns were independent of patients' age, height, and weight.
Power analysis suggested that a sample size of 13 end-stage COPD patients would provide 90% power to detect a difference of 1 SD (effect size of 1.0) in each outcome variables comparing pre-and post-TEA.
Thirteen patients were initially selected; however, one patient was subsequently eliminated because anesthesia spread was considered insufficient after 45 minutes.
All remaining 12 patients exhibited anesthesia spread from T2-8.
This final sample size (12 patients) reduced the study power to 88% which was considered adequate. The P values were two-tailed with statistical analysis performed using SPSS software.
The table above provides values concerning preoperative vital capacity, age, FEV1, and residual volume. Furthermore, the mean spread of epidural blockade was 8.2 +/- 1.3 segments with a minimum caudal block extent being T8. As noted above, one patient was an anesthetic spread of only five segments was excluded. The table below characterizes changes in breathing pattern:
|
|
VT (tidal volume), Rf (respiratory frequency),VE (minute ventilation) and Ti/Ttot (duty cycle), factors the described breathing pattern are reported in table 2.VT andVE were significantly increased after TEA, mean RL was 20.7 +/- 9.9 cm H2Ol/sec. pre-TEA, compared to the significant decrease of 16.6 +/- 8.1 cm H2O/l/sec as noted in table 3.
RL pulmonary resistance
VT tidal volume
VE minute ventilation
Ti/Ttot duty cycle; inspiration duration (TI)/ expiration duration (TE)
PIFR increased from 0.48 +/- 0.17 L/s to 0.55 +/- 0.14 L/s, although PEFR was unchanged
PIFR: Peak inspiratory flow rate
PEFR: Peak expiratory flow rate
Changes in PIFR and RL were not dependent upon age, height or weight
WOB, PEEP i,dyn and
Pes were not altered pre- vs post-TEA.
PaO2: not affected by TEA
PaCO2: slightly decreased by TEA
MIP: unchanged by TEA
|
|
The authors begin the discussion section by noting that the purpose of the experiment was to assess TEA effects with bupivacaine 0.25% in patients with end-stage COPD.
The conclusion was that TEA did not negatively affect ventilatory mechanics, breathing pattern, gas exchange, and inspiratory muscle force generation in these 12 patients.
All subjects served as their own control, an approach designed to overcome any possible interindividual differences.
Therefore, ventilatory mechanics, in each patient, was assessed both before and TEA. The bupivacaine 0.25% was chosen over bupivacaine 0.5% since the latter concentration was thought more likely to cause expiratory muscle paralysis whereas the 0.25% dosage would more likely favor sensory blockade.
So in summary, following baseline patient measurements, bupivacaine was administered using the thoracic epidural catheter and TEA measurements performed about 45-60 minutes later.
This procedure was used in all patients.
The Bicore monitoring system has been found suitable for application in patients with severe COPD.
However, the authors note that changes in respiratory muscle activity during expiration was not directly assessed using electromyography.
This limitation had to do with ethical concerns.
Alteration in expiratory muscle activity could influence the variables characterizing respiratory mechanics-variables including WOB, PEEP i,dyn and RL . In order to determine if the study method was reflecting changes in expiratory muscle activity, the following approach was utilized.
For 6-10 breaths (consecutive) to airflow,V, Paw and
Pes were evaluated in
addition to the
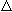 Pes
for 39 consecutive breaths. No differences in
Pes
for 39 consecutive breaths. No differences in
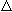 Pes
was observed comparing pre-TEA and post-TEA measurements.
Unchanged
Pes
was observed comparing pre-TEA and post-TEA measurements.
Unchanged
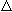 Pes
and PEEP i,dyn
values at 2 time points was considered as reasonable
indirect evidence that changes in expiratory muscle activation
did not influence study results.
Pes
and PEEP i,dyn
values at 2 time points was considered as reasonable
indirect evidence that changes in expiratory muscle activation
did not influence study results.
Also, the observed increases in VT and VE following TEA would have been unanticipated results if in fact respiratory muscles were inactivated. Also, inspiratory muscle activity itself, evaluated by MIP values, were unchanged. All this evidence points to results unaffected by changes in expiratory muscle activity; however, this possibility cannot be completely eliminated unless direct myographic measurements had been obtained.
It might initially appears surprising that only 12 individuals were used in the study. Increased power might be obtained by doubling or even tripling this number.
The authors confront this issue directly by indicating that the patients chosen, 12 end-stage COPD patients about to undergo lung volume reduction surgery exhibit extreme respiratory limitation as well as frequent anxiety attacks.
Because of the fragility of this patient population, the authors considered it prudent to use the smallest possible sample size that would reasonably be expected to detect clinically relevant differences.
Within this framework and using this sample size, the power to detect a difference of 1 SD in each outcome variables between pre-and post TEA was 88% which was considered sufficient for detecting clinically important differences.
Detailed consideration of specific results was presented in the paper's Discussion section, but will not be reviewed here.
However, the final conclusion was that following TEA, a decrease in RL along with an increase in PIFR and VE with unchanged dynamic pulmonary hyperinflation and WOB in end-stage COPD patients, were observed.
Also, TEA did not influence inspiratory muscle force. As a result of these findings, TEA (bupivacaine 0.25%) did not cause unfavorable changes in ventilatory mechanics, inspiratory muscle force generation or gas exchange in advanced COPD subjects.
Therefore, in this especially at-risk patient subgroup TEA (bupivacain 0l25%) seems to be safe to use.