|
|
Anesthesia Pharmacology Chapter 30: Congestive Heart Failure
Clinical Manifestations and Physical Findings in Congestive Heart Failure
Dyspnea: The most common symptom of heart failure, dyspnea or respiratory distress occurs because of increased effort of breathing.
Dyspnea occurs initially upon exertion, but in advanced CHF may occur at rest.
Cardiac dypsnea is usually seen in patients with increased pulmonary venous and capillary pressure.
The combination of interstitial pulmonary edema and vessel enlargement result in reduced lung compliance and increased respiratory muscle work of breathing.
Enhanced activity of respiratory muscles further compromise available oxygen and contributes to the sensation of breathlessness.
Orthopnea, dyspnea while recumbent, is due fluid redistribution from the lower extremities into the chest with a resultant increase in pulmonary capillary hydrostatic pressure.
Paroxysmal (nocturnal) dyspnea is a sudden-onset of severe shortness of breath and coughing, awakening the patient.
Factors that produce paroxysmal dyspnea include:
depression of respiratory center during sleep (decreases arterial oxygen)
decreased ventricular function due to decreased sympathetic tone (decrease myocardial contractility and hence cardiac output) and
redistribution of fluid to the chest.
Acute pulmonary edema: severe form of cardiac asthma
|
|
Initially, the edema is found in the lower lobes; however, in advanced disease all lobes may be involved.
Sectioning of the lobes reveals a sanguineous fluid consisting of a mixture of air and edema fluid.
Figure and description adapted from "Robbins: The Pathological Basis of Disease" Fifth Edition, p. 96
|
|
Pulmonary edema is common in congestive heart failure.
As pulmonary capillary pressures increase, the initial fluid excess is removed by increased lymphatic drainage.
When lymphatic system capacity is exceeded, pulmonary edema occurs.
Radiographic signs include septal lines, bronchial wall thickening and subpleural pulmonary edema.
This radiograph illustrates generalized fissural thickening and lack of clarity of intrapulmonary vessels and septal lines.
Figure and description from "Imaging Diseases of the Chest" , p. 388, by Peter Armstrong, Alan G. Wilson, and Paul Dee, Yearbook Medical Publishers, Inc. 1990.
Cardiac asthma, related to paroxysmal dyspnea, is characterized by wheezing due to bronchospasm.
Acute pulmonary edema, however, involves a significant elevation of pulmonary capillary pressure leading to alveolar edema, extreme shortness of breath and rales.
Expectoration of blood-tinged fluid may occur.
![]() Acute pulmonary edema may be fatal.
Acute pulmonary edema may be fatal.
Fatigue, weakness and reduced exercise capacity are common in congestive heart failure patients.
Exercise capacity is reduced because increased cardiac output required to support increased levels of physical activity is unavailable or inadequate.
Pulmonary Rales
Peripheral (cardiac) edema is commonly seen symmetrically in the legs, particular in the pretibial region and ankles.
|
|
|
Ascites and pleural effusion.
Pleural effusion results from increased pulmonary capillary hydrostatic pressure and the attendant movement of fluid into the pleural cavity.
Congestive Hepatomegaly
Jaundice
|
increased circulating tumor necrosis factor |
increased metabolic rate need to support the increased effort of breathing |
anorexia, nausea, vomiting due to digitalis intoxication, congestive |
|
hepatomegaly, and abdominal fullness |
impairment of intestinal absorption due to intestinal venous congestion |
protein-losing enteropathy (rare) |
|
The fundamental abnormality in heart failure is embodied in:
|
|
Figures from "Harrison's Principles of Internal Medicine", Thirteenth Edition, pages 995 and 996. |
|
Factors Influencing Cardiac performance and Output
Ventricular end-diastolic volume (preload)
Atrial contraction
Inotropic state (myocardial contractility)
Ventricular afterload
Exercise
Ventricular end-diastolic volume (preload)
![]() For any given inotropic state,
ventricular performance will be significantly
affected by the degree of ventricular stretch as
determined by ventricular end-diastolic volumes (EDV).
For any given inotropic state,
ventricular performance will be significantly
affected by the degree of ventricular stretch as
determined by ventricular end-diastolic volumes (EDV).
The general relationship, as shown above, is the Frank-Starling mechanism.
Several factors influence end diastolic volumes, beginning with total blood volume.
Significant volume depletion leads to decreased cardiac output with preload and end-diastolic volumes declining (NPO, fluid replacement).
Blood volume distribution is important. Factors that affect distribution include:
Body position: gravitational forces, in the upright individual, results in pooling to extrathoracic compartments, especially the legs.
By contrast, in the recumbent individual, blood will redistribute into the thorax, increasing preload.
Venous tone:
Venular smooth muscle tone is under sympathetic neural and humoral control.
Exercise or hypotension result in venoconstriction and an increase in intrathoracic, intraventricular blood volume.
Accordingly, these conditions increase cardiac output by increasing end-diastolic volumes.
Skeletal muscle activity: During exercise, blood is displaced from the periphery due to the squeezing action of contracting muscle on the venous bed. Increased preload results as does increased cardiac output and work.
Atrial contraction
Atrial contraction (atrial kick) enhances ventricular filling.
In the hypertrophic ventricle (with reduced compliance) appropriately timed atrial contraction may be especially important in achieving adequate levels of ventricular filling.
Increased heart rate (reduced filling times) or atrial arrhythmias reduce or eliminate the effectiveness of atrial kick.
Inotropic state (myocardial contractility)
Factors that influence the inotropic state affect ventricular performance at a given ventricular end-diastolic volume.
These factors change the concentration of Ca2+ at the myofilaments and include in part:
Adrenergic nerve activity: The amount of norepinephrine released by cardiac adrenergic nerve endings is the most important acute factor in changing the position of the force-velocity in ventricular function curves.
Circulating catecholamines: Catecholamines released from adrenal medulla and other non-cardiac sympathetic ganglia stimulate cardiac adrenoceptors increasing rate and force of contraction.
Exogenously administered agents:
|
cardiac glycosides |
dopamine (Intropin)/dobutamine (Dobutrex) |
caffeine |
|
isoproterenol (Isuprel) |
theophylline |
calcium |
Exogenously administered agents:
|
procainamide (Procan SR, Pronestyl-SR) |
disopyramide (Norpace) |
certain calcium channel blockers |
|
alcohol |
barbiturates |
local and general anesthetics |
Physiological Depressants:
Depression of the myocardial force-velocity curves with decreased left ventricular function can occur with hypoxia, hypercapnia, ischemia, and acidosis.
Loss of ventricular muscle mass:
When significant portions of the ventricular are hypokinetic or necrotic either due to ischemia or following myocardial infarction, total ventricular performance may be significantly depressed.
Ventricular afterload
Afterload is the stress developed in the wall of the ventricle during ejection and depends on aortic pressure and ventricular dimensions.
Myocardial fiber tension is determined by the product of the intracavity ventricular pressure and radius divided by wall thickness (Laplace's law).
Therefore, for the same level of aortic pressure, afterload increases with ventricular dilation.
Left ventricular stroke volume is inversely proportional to afterload.
P = (T * M)/R
|
![]() In the failing heart with limited
or no preload reserve (increasing preload in a
normal heart increase contractility), afterload
determines ventricular performance.
In the failing heart with limited
or no preload reserve (increasing preload in a
normal heart increase contractility), afterload
determines ventricular performance.
When afterload increases (increase in vasoconstriction) in the failing heart, cardiac output may be reduced further even while oxygen demand increases.
Vasodilators may improve myocardial performance by reducing ventricular afterload.
With exercise, venous return is significantly increased and results in enhanced ventricular filling and preload.
Increases in cardiac adrenergic activity and increases in circulating levels of catecholamines increase heart rate and enhance the myocardial contractility.
These factors result in significantly augmented cardiac output.
Arterial pressure does not increase substantially since vasodilatation in exercising muscles offset the increase in cardiac output.
Myocardial Adaptation including Neurohumoral Adjustments
Adaptive mechanism to assist the failure heart
Frank-Starling relationship: Increasing preload leads to increased cardiac output.
Myocardial hypertrophy which tends to reduce ventricular wall tension towards normal.
Redistribution of cardiac output from skin, kidneys, and skeletal muscle to the brain and heart.
Neurohumoral adjustments which maintain arterial pressure
Renin-Angiotensin System (RAS): Activation of the RAS occurs with declining cardiac output.
Increasing concentrations of circulating angiotensin II and aldosterone results in excess vasoconstriction and salt and water retention respectively.
The clinical condition of patients with chronic heart failure may be improved by administration of aldosterone antagonists and ACE inhibitors.
Adrenergic System: Patients with heart failure often have significantly elevated circulating norepinephrine levels.
These levels provide critical inotropic support for the failing myocardium.
![]() Administration of
beta-adrenoceptor
antagonists to patients with
severe failure may worsen their congestive
heart failure.
Administration of
beta-adrenoceptor
antagonists to patients with
severe failure may worsen their congestive
heart failure.
![]() Administration of beta-adrenoceptor antagonists
to patients with mild to moderate failure
may be helpful in management of their
congestive heart failure
Administration of beta-adrenoceptor antagonists
to patients with mild to moderate failure
may be helpful in management of their
congestive heart failure
Congestive Heart Failure: Causes
Arrhythmias: In patients with heart disease and with a history of congestive failure, an acute arrhythmia is a common precipitating cause of CHF.
|
|
|
|
Myocardial Infarction: A myocardial infarction, reducing left ventricular function, may precipitate CHF in a previously hemodynamically compensated patient.
Pulmonary Embolism: Physically inactive patients with low cardiac output may develop deep venous thrombi which may produce pulmonary emboli and elevation of pulmonary arterial pressure. Increased pulmonary artery pressure may worsen or cause left ventricular failure.
Systemic Hypertension: Rapid increases in arterial blood pressure with associated increases in peripheral resistance can increase afterload to an extent sufficient to produce heart failure.
*Specific CHF Causes
Thyrotoxicois
Pregnancy
Infection
Anemia
Rheumatic and other forms of Myocarditis
Infective Endocarditis
Physical, dietary, fluid, environmental and emotional excesses
*Isselbacher et al. (eds): "Harrison's Principles of Internal Medicine"New York, McGraw-Hill Inc, 1994, p. 999.
Receptor polymorphism
Genetic variation that may affect responses by individuals to physiological stresses or to pathophysiological conditions may include differences in the molecular detail of prominent receptors important in myocardial function.
An example of genetically-directed structural variation due to polymorphism in the genetic coding block is the beta2 adrenergic receptor system.
The beta2 adrenergic receptor (7 transmembrane components) can be represented in a manner that illustrates the locations of amino acid substitutions which define the polymorphism within the human population:
|
|
|
Focusing uncertain amino acids present in the wild type altered in the polymorphic phenotype, note:
|
Wild type |
Structure |
|
structure |
|
arginine 16 (Arg16) |
|
glycine 16 (Gly16) agonist promoted down-regulation enhanced |
|
|
glutamine 27 (Gln27) |
|
glutamate 27 (Glu27) agonist-promoted down-regulation absent |
|
|
Valine 34 (Val34) |
|
Methionine 34 (Met34) no difference compared to wild type |
|
|
Threonine 164 |
|
Isoleucine 164 uncoupled from stimulatory G protein |
|
Beta2 adrenergic receptor polymorphisms appear important in the pathophysiology congestive heart failure.
For example, Liggett2noted that in comparing clinical courses in 259 patients with idiopathic dilated or ischemic cardiomyopathy, survival of individuals with the isoleucine164 substitution for the normally occurring threonine164 was notably altered. Specifically, the one-year survival was 42% (Ile164)compared to 76% (threonine164, i.e.wild type). Relative death risk or cardiac transplantation: 4.81 [p < 0.001].
The magnitude of these effects may be appreciated in the comparison of the heart from two patients exhibiting position164 genetic polymorphisms:
|
|
|
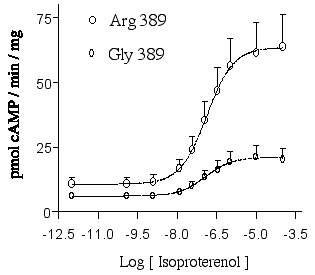
Concerning the beta1 adrenergics system, genetic polymorphism has also been noted. For example at position 389, glycine or arginine can be present, with arginine more common. This region is associated probably with G-protein coupling, see below:
|
|
|
The polymorphism that position 389 appears to also vary based on race3. Furthermore, a difference in the coupling to adenylyl cyclase has been observed:4
Comparative pathophysiology: diastolic vs. systolic heart failure
5Congestive heart failure is often thought of in terms of a failure of left ventricular contractile function.
Left ventricular failure may occur for a variety of reasons including but not limited to valvular dysfunction or as the consequences significant loss of left ventricular myocardial muscle mass.
![]() Interestingly, myocardial-induced dyspnea
or fatigue may occur in the absence of abnormal contractility.
Interestingly, myocardial-induced dyspnea
or fatigue may occur in the absence of abnormal contractility.
In these cases, the pathophysiological process may be associated with abnormalities in diastole, for instance those that are manifest as inadequate left ventricular filling.
![]() 5Heart
failure due to diastolic abnormalities exhibit a frequency of about
14%-41%, depending on the study6-9,
with diastolic dysfunction resulting in failure probably more prevalent
in elderly patients9.
5Heart
failure due to diastolic abnormalities exhibit a frequency of about
14%-41%, depending on the study6-9,
with diastolic dysfunction resulting in failure probably more prevalent
in elderly patients9.
Generally,left ventricular diastolic function normally declines with age despite there being no specific age-related abnormality in contractility.
Elderly patients may however be more likely to experience factors that cause enhanced ventricular stiffness or limit ventricular relaxation.
These factors include: ischemia, hypertension and/or tachycardia (systolic hypertension is one factor that clearly increases in likelihood with advancing age)
1What happens during diastole? Physiological issues:
The myocardial contraction-relaxation cycle centers around fairly rapid changes in free calcium concentration. The stepwise process involves:
membrane depolarization promoting myocyte Ca2+ entry through slow (L-type) Ca2+ channels
this initial process causes significant additional sarcoplasmic reticular Ca2+ release
Ca2+ interaction with troponin leads to subsequent promotion of actin-myosin interactions and muscle contraction..
Relaxation can only occur rapidly if the free calcium is rapidly removed.
Calcium transport for purpose of establishing the basal state occurs through the action of a calcium-ATPase, which handles up to 90% of free calcium by re-storage back into the sarcoplasmic reticulum. The remaining 10% is removed through Na+/Ca2+ exchange mechanisms and other mechanisms.
|
|
|
The relaxation phase of the cardiac cycle: This phase consists of 4 components:
isovolumic relaxation
rapid filling
slow filling (diastasis)
atrial contraction
|
|
|
1Looking at each phase separately, the first phase is defined by the time between aortic valve closing and mitral valve opening. From the ionic point of view, during this time free calcium is translocated back into the sarcoplasmic reticular storage sites.
The next phase is initiated when left ventricular pressure falls below left atrial pressure; the pressure differential drives mitral valve opening with attendant left ventricular filling. This process continues until pressure equilibration.
Although only about 30 percent of the diastolic time occurs during phase 2, typically up to 80% of left ventricular filling occurs during the stage.
Therefore, the next phase is referred to as a slow-filling time; the filling is mainly pulmonary vein flow [this phase is most sensitive to an increased heart rate, i.e. reduced filling]
Atrial contraction is the last component and typically contributes about 15%-25% of left ventricular diastolic volume-this amount would nominally be referred to as the "atrial kick". However, with reduced left ventricular compliance this phase is more important, perhaps contributing up to 40%.
Pathophysiological issues:
1With diastolic dysfunction, there is both elevated left ventricular filling pressure and elevated pulmonary venous pressure. To maintain cardiac output under load, higher filling pressures are required to achieve adequate left ventricular filling.
![]() However, in patients with diastolic dysfunction,
increased left ventricular filling pressure did not result in
increased left ventricular diastolic volumes13.
However, in patients with diastolic dysfunction,
increased left ventricular filling pressure did not result in
increased left ventricular diastolic volumes13.
|
|
|
Diastolic heart failure may develop slowly and occur as a result those long-term consequences of compensatory physiological responses.
Factors that may induce diastolic functional pathologies include increased heart rate, left ventricular hypertrophy, and myocardial ischemia. Some of these factors may be associated with progressive myocardial "remodeling"
Recall that the key to rapid myocardial relaxation is the ability to re-sequester free Ca2+ . Furthermore, this process requires ionic translocation against a significant concentration gradient (10,000: 1) and as such is coupled to ATP hydrolysis.The ratio is 1 ATP hydrolyzed for every two Ca2+ translocated by the calcium-ATPase pump.
Therefore, factors the decrease ATP availability will impair this translocation process.
Myocardial hypertrophy and ischemia reduce ATP availability and can be therefore readily identified as contributing pathologic factors that impair myocardial relaxation.
Additionally, hypertrophic ventricular muscle tissue exhibits reduced compliance (increased stiffness)
1Factors that impair ventricular contraction include:
hypertrophy, ischemia, hypertension, collagen deposition and fibrosis, regional asynchrony, increase preloaded and afterload, intrinsic abnormalities in calcium movement, and tachycardia
Factors that reduce ventricular compliance (increased stiffness) include:
hypertrophy, hypertension, collagen deposition and fibrosis, pericardial constriction or restriction.
Aging is associated with increased likelihood of left ventricular hypertrophy and/or ischemia.
Aging is also associated with increased collagen deposition which reduces ventricular compliance. However other important causes those diastolic failure include coronary vascular disease, hypertension, diabetes, obesity, and aortic stenosis
1Pharmacological interventions: Overview:
Many different drug categories at least in principle might be thought to improve isolated diastolic dysfunction and therefore improve exercise tolerance and reduce other symptoms as well as increase left ventricular filling rate and reduce heart rate.
Drug categories that have been evaluated include:
calcium channel blockers
beta adrenergic receptor antagonists
angiotensin converting enzyme inhibitors
diuretics
nitrates
![]() 1Presently,
isolated diastolic heart failure is managed with many of the above
agents.
1Presently,
isolated diastolic heart failure is managed with many of the above
agents.
The rationale by which calcium channel blockers might improve diastolic heart failure include:
direct effects on calcium movements
indirect effects through blood pressure reduction, reducing myocardial ischemia, decreasing (promoting regression of) left ventricular hypertrophy, decreasing heart rate {for verapamil (Isoptin, Calan) and diltiazem (Cardiazem)} thereby improving left ventricular filling.
1Weinberger, H., Diagnosis and Treatment of Diastolic Heart Failure, Hospital Practice http://www.hosppract.com/issues/1999/03/weinb.htm
2Liggett SB, Wagoner LE, Craft LL, Hornung RW, Hoit BD, McIntosh TC, Walsh RA. The Ile164 beta2-adrenergic receptor polymorphism adversely affects the outcome of congestive heart failure. J Clin Invest 102:1534-1539, 1998.
3Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac beta1-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C. Human Mutation 14(3):271, 1999.
4Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem 274:12670-12674, 1999.
5Spencer, K.T. and Lang, R.M. Kirk T. Spencer, MD Roberto M. Lang, MD, Diastolic heart failure, What primary care physicians need to know , vol. 101, no. 1, January 1997, Postgraduate medicine, http://www.postgradmed.com/issues/1997/01_97/spencer.htm
6Aronow WS, Ahn C, Kronzon I. Prognosis of congestive heart failure in elderly patients with normal versus abnormal left ventricular systolic function associated with coronary artery disease. Am J Cardiol 1990;66(17):1257-9
7Takarada A, Kurogane H, Minamiji K, et al. Congestive heart failure in the elderly-echocardiographic insights. Jpn Circ J 1992;56(6):527-34
8.Iriarte M, Murga N, Sagastagoitia D, et al. Congestive heart failure from left ventricular dysfunction in systemic hypertension. Am J Cardiol 1993;71(4):308-312
9.Madsen BK, Hansen JF, Stokholm KH, et al. Chronic congestive heart failure: description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 1994;15(3):303-10
10Iwase M, Nagata K, Izawa H, et al. Age-related changes in left and right ventricular filling velocity profiles and their relationship in normal subjects. Am Heart J 1993;126(2):419-26
11Klein AL, Burstow DJ, Tajik AJ, et al. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc 1994;69(3):212-24
12Zile MR: Diastolic dysfunction: Detection, consequences, and treatment. Part I: Definition and determinants of diastolic function. Mod Concepts Cardiovasc Dis 58:67, 1989
13Kitzman DW et al: Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the Frank-Starling mechanism. J Am Coll Cardiol 17:1065, 1991
General References
Hollenberg, S.M. and Parrillo, J.E., Shock, In Harrison's Principles of Internal Medicine 14th edition, (Isselbacher, K.J., Braunwald, E., Wilson, J.D., Martin, J.B., Fauci, A.S. and Kasper, D.L., eds) McGraw-Hill, Inc (Health Professions Division), 1998, p. 215-222
Hoffman, B.B and Lefkowitz, R.J, Catecholamines, Sympathomimetic Drugs, and Adrenergic Receptor Antagonists, In, Goodman and Gillman's The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) The McGraw-Hill Companies, Inc.,1996, pp.222-224.
Stoelting, R.K., "Sympathomimetics", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, p.259.