-
Halothane (Fluothane) 
-
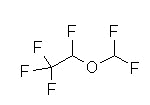
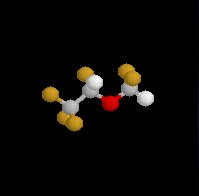
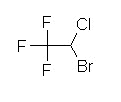
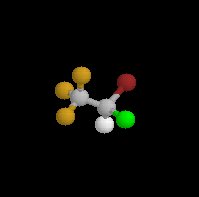
Halothane (Fluothane), a nonflammable liquid at room temperature is a halogenated alkane derivative.C2-H-Br-Cl-F3
-
-
Halothane (Fluothane) vapor has been described as having a sweet, non-pungent odor which made it and agent of choice among the older inhalational agents for pediatric anesthesia.
-
Other inhalational agents are effective in anesthesia maintenance both for infants and children; however, most of these agents have sufficiently unpleasant, pungent odor that they should not be used for inhaled induction. (isoflurane (Forane) and desflurane (Suprane) are examples of agents which may be used in pediatric cases for anesthesia maintenance, but because of their odor they really cannot be used for mask induction.
-
Concerns about halothane (Fluothane)'s use in children have to do with the following hemodynamic characteristics:
-
Halothane (Fluothane) is a myocardial depressant, an effect which is particularly apparent in children, especially in hypovolemic patients.
-
The extent of cardiac depression can be reduced if halothane (Fluothane) requirements are reduced by concurrent administration of other agents such as opioids or muscle relaxants.
-
-
Halothane (Fluothane) can sensitize the myocardium to catecholamines, thus predisposing towards arrhythmias. This effect is more likely to occur during "light" anesthesia or in the presence of hypercarbia. When epinephrine is required, up to 10 μg/kg may be given in the normocarbic pediatric patients without incurring significant risk.
-
-
Alternatives to halothane (Fluothane) for mask induction in pediatric patients: Sevoflurane (Sevorane, Ultane), an inhalational agent with a non-pungent odor, is probably the agent of choice for pediatric patients.
-
Sevoflurane (Sevorane, Ultane) combines both rapid induction and emergence, secondary to its low lipid solubility, with very low myocardial depression -- even at high vaporizer output.
-
Furthermore, there probably is a reduced likelihood of cardiac arrhythmias with sevoflurane (Sevorane, Ultane) compared halothane (Fluothane) in this patient group.
-
-
-
Halothane (Fluothane), a nonflammable relatively potent inhalational agent exhibits a low blood:gas partition coefficient, predicting relatively rapid induction and recovery
-
This agent can be used to provide controlled hypotension bleeding control
-
Intermediate solubility (blood) + high-potency result in rapid onset/recovery from anesthesia (either alone or in combination with nitrous oxide or injected opioids)Chemical Considerations: (see structure above)
-
Consequences of halogenated structure:
-
Nonflammability
-
Intermediate blood solubility
-
Anesthetic potency
-
Molecular stability
-
Carbon-fluorine: decreased flammability
-
Trifluorocarbon: molecular stability
-
Carbon-chlorine and carbon-bromine components (with retention of a hydrogen atom) contributing to anesthetic potency
-
Decomposition concerns:
-
Susceptible to decomposition to: HCl, hydrobromic acid, bromide and phosgene.
-
Amber-colored bottle storage + thymol (reduces spontaneous oxidative decomposition)
-
Thymol: remaining in vaporizers, following halothane (Fluothane) vaporization leading to malfunction of temperature-compensating devices or vaporizer turnstiles
-
-
-
-
Halothane (Fluothane) anesthesia: provides unconsciousness
-
 Clinical
Indication:
Clinical
Indication:-
Halothane (Fluothane) is effective for general anesthetic maintenance and may be an induction agent of choice in difficult airway
-
-
Systems Physiology:
-
CNS:
-
Generalized CNS depression
-
Cerebrovascular dilation causes increased ICP
-
-
Cardiovascular:
-
Halothane (Fluothane) causes a slight decrease in heart rate, a decrease in mean arterial pressure (MAP)-- both associated with its well-documented cardiac depressant property.
-
Halothane (Fluothane) may also sensitize the myocardium to circulating catecholamines, explaining the pro-arrhythmogenic property.
-
-
Pulmonary:
-
Halothane (Fluothane) causes decreasing tidal volume with increasing MAC, increasing respiratory rate with increasing MAC
-
Halothane (Fluothane) also promote significant bronchodilation
-
-
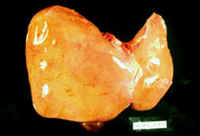
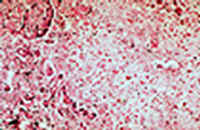
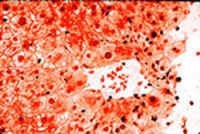
 Hepatic: fulminant hepatic failure (halothane hepatitis):
Hepatic: fulminant hepatic failure (halothane hepatitis):
-
Case history for slides below: "Forty four year old lady had uterine curettage for metrorrhagia. Eight weeks later she had hysterectomy.
-
Three days after hysterectomy she became jaundiced.
-
Seven days later she died in liver failure.
-
Halothane was used as anesthetic in both surgical procedures.
-
The autopsy showed massive liver necrosis. It appears that the liver damage was mediated by an immunoreactive mechanism. Multiple exposures increase the incidence of liver damage." DRUG-INDUCED LIVER INJURY by Dr. Emilio Orfei
-
-
|
|
|
|
|
-
Musculoskeletal: relaxation
-
 Contraindications:
Contraindications:-
Trigger to malignant hyperpyrexia
-
Recent halothane (Fluothane) exposure
-
-
Drug-drug interactions:
-
Muscle relaxants potentiation
-
Reduced MAC with opioids, nitrous oxide and benzodiazepines
-
-
Concerns:
-
Inadequate analgesia
-
May not provide adequate muscle relaxation
-
May not provide adequate suppression of visceral reflexes
-
Reversible reduction of GFR
-
-
Halothane Disadvantages
-
Unpredictable occurrence of hepatitis
-
Status: infrequently used due to the availability of isoflurane (Forane), enflurane (Ethrane) and desflurane (Suprane).
-
Kennedy, S.K. and Longnecker, D.E., History and Principles of Anesthesiology In, Goodman and Gillman's The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) TheMcGraw-Hill Companies, Inc.,1996, p 313.
White, P. F. "Anesthesia Drug Manual", W.B. Saunders Company, 1996, p. 249.
-
-
-
Overview: Low-molecular weight
-
Oderless to sweet smelling
-
Nonflammable
-
Low-potency gas
-
Poor blood solubility (0.46)
-
Low blood solubility allows rapid attainment of alveolar and brain partial pressure
-
-
-
 Most commonly
administered in combination with opioids or
volatile anesthetics.
Most commonly
administered in combination with opioids or
volatile anesthetics.-
With a MAC value of 105%, nitrous oxide, by itself is not suitable or safe as a sole anesthetic agent.
-
-
Effective analgesic.
-
Minimal skeletal muscle relaxation
-
Effective: Nitrous oxide in combination with thiopental for induction, a skeletal muscle relaxant, and hyperventilation to reduce CO2
-
Nitrous oxide can be used as an adjunct to other agents: For example, using 70% nitrous oxide + oxygen significantly reduces MAC for halothane, enflurane, and isoflurane.
-
Despite the relative insolubility of nitrous oxide, large quantitities of gas are rapidly absorbed due to its high inhaled concentration.
-
This concentration effect speeds induction as fresh gas is literally drawn into the lung from the breathing circuit.
-
-
 Since nitrous oxide is
often administered with a second gas, the second
gas effect also enhances the rate of induction
Since nitrous oxide is
often administered with a second gas, the second
gas effect also enhances the rate of induction -
 If administration of nitrous oxide
is abruptly discontinued, rapid transfer of NO
from blood and tissues to the alveoli decreases
arterial tension of oxygen.
If administration of nitrous oxide
is abruptly discontinued, rapid transfer of NO
from blood and tissues to the alveoli decreases
arterial tension of oxygen.
-
This process is diffusional hypoxia.
-
-
Nitrous oxide
-
Nitrous oxide should not be used if pockets of trapped air are suspected in the patient (e.g. following a pneuomoencephalogram or in an occluded middle ear, because of exchange of NO with nitrogen with attendant gas expansion.
-
This observation should not be interpreted to suggest that nitrous oxide does not play a significant role in otolaryngologic surgical procedures. For example, in tonsillectomy and adenoidectomy, induction is typically with a volatile agent + nitrous oxide. Furthermore, with myringotomy and tube insertion a volatile agent and nitrous oxide can be used.
-
For tympanoplasty, however, nitrous oxide should be discontinued during the placement of the tympanic membrane graft
-
-
Nitrous oxide has minimal effects on the circulation compared to the other inhalational agents with which it is co-administered.
-
May inactivate vitamin B12.
-
Nitrous oxide by itself has minimal effects on respiratory drive.
-
Minimal skeletal muscle relaxation.
-
No significant effects on the liver, kidney, or GI tract.
-
-
 Nitrous Oxide Disadvantages
Nitrous Oxide Disadvantages-
No skeletal muscle relaxation
-
Weak anesthetic
-
Air pockets in closed spaces expand
-
Post-anesthesia hypoxia (diffusion hypoxia)
-
Not suitable as a sole anesthetic agent
-
Kennedy, S.K. and Longnecker, D.E., History and Principles of Anesthesiology In, Goodman and Gillman's The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) The McGraw-Hill Companies, Inc.,1996, p 319 - 321.
Stoelting, R.K., "Inhaled Anesthetics", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, pp 36-76
-
-
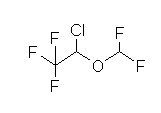
Desflurane (Suprane) Overview:
-
-
Fluorinated methyl ethyl ether and, similar to isoflurane (Forane). Substitution of a fluorine atom for chlorine (isoflurane)
-
Fluorination (compared to chlorination) results in:
-
Increased vapor pressure-- 3X (= decreased intermolecular attraction)
-
High vapor pressure would result in boiling at operating room temperatures requires:
-
Implementation of new vaporizer technology converts desflurane (Suprane) to a gas (heated and pressurized electric vaporizer) which is blended with fresh gas flow
-
-
-
Increased molecular stability
-
Decreased potency: 5 fold < isoflurane (Forane)
-
Minor desflurane (Suprane) metabolism
-
Pungent odor: Desflurane (Suprane) less likely to be used for inhalation induction compared to halothane (Fluothane) or sevoflurane (Sevorane, Ultane).
-
Airway irritation, breath-holding, coughing, laryngospasm is greater than 6% desflurane (Suprane) administered to an awake patient.
-
Significant salivation
-
Carbon monoxide:
-
Secondary to desflurane (Suprane) degradation by strong base present in carbon dioxide absorbants.
-
Carbon monoxide concentration: desflurane (Suprane) > enflurane (Ethrane) > isoflurane (Forane) (carbon monoxide produced by halothane (Fluothane) or sevoflurane (Sevorane, Ultane): extremely small)
-
Solubility (blood: gas partition coefficient = 0.45) and potency (MAC 6%)
-
Rapid achievement of alveolar partial pressures required for anesthesia along with rapid awakening
-
Distinguishing features of desflurane (Suprane) and sevoflurane (Sevorane, Ultane) compared to earlier volatile anesthetics:
-
Lower blood-gas solubility
-
More rapid recovery from anesthesia
-
-
-
Desflurane (Suprane) Systems Physiology:
-
CNS:
-
Generalized depression
-
Extremely rapid emergence
-
Increased ICP
-
-
Cardiovascular:
-
Vascular resistance
-
MAP
-
Heart rate (deep anesthesia); tachycardia with rapid concentration change
-
-
Pulmonary:
-
Decrease in tidal volume
-
Increase in respiratory rate
-
Irritant
-
-
Gastrointestinal: nausea
-
Musculoskeletal:relaxation
-
-
 Desflurane (Suprane): Clinical Indications:
Desflurane (Suprane): Clinical Indications:-
General anesthesia maintenance
-
Agent of choice when:
-
Rapid emergence desirable
-
Precise anesthetic depth control required
-
-
-
 Contraindications:
Contraindications:-
Trigger to malignant hyperpyrexia
-
-
Drug-drug interactions:
-
Muscle relaxant effect potentiation
-
MAC with opioids, nitrous oxide, and benzodiazepines
-
Stoelting, R.K., "Inhaled Anesthetics", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, pp 36-76
White, P. F. "Anesthesia Drug Manual", W.B. Saunders Company, 1996. p.248
-
-
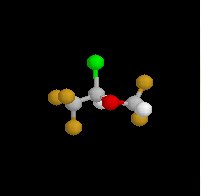
Isoflurane (Forane) Overview:
-
-
-
Halogenated methyl ethyl ether
-
Non-flammable liquid (room temperature);pungent odor; ether-like
-
Intermediate solubility (blood) + high-potency ® rapid onset and recovery using isoflurane (Forane) alone or in combination with nitrous oxide or opioids (injected)
-
Very high physical stability; no need to add preservatives e.g. thymol
-
-
 Isoflurane anesthesia:
results in unconsciousness
Isoflurane anesthesia:
results in unconsciousness-
Initially, until deeper levels of anesthesia are reached, isoflurane stimulates airway reflexes with:
-
Increases in secretions
-
Coughing
-
Laryngospasm. (greater with isofluorane than enflurane (Ethrane) or halothane (Fluothane))
-
-
-
Isoflurane (Forane): Systems Physiology:
-
CNS:
-
Generalized CNS depression; Rapid emergence
-
Increased ICP
-
-
Cardiovascular:
-
Little effect on cardiac output and decreased vascular assistance and decreased MAP
-
Increased heart rate
-
-
Gastrointestinal: nausea
-
Musculoskeletal: relaxation
-
-
 Contraindications: trigger to malignant hyperpyrexia
Contraindications: trigger to malignant hyperpyrexia -
Drug-drug interactions:
-
Muscle relaxants potentiation
-
MAC with opioids, nitrous oxide, and benzodiazepines
-
-
Isoflurane (Forane) Comparative Pharmacology
-
By contrast to enflurane (Ethrane) or halothane (Fluothane) cardiac output is well maintained with isoflurane (Forane)
-
May provide adequate muscle relaxation greater than seen with halothane (Fluothane);
-
Perhaps adequate for abdominal procedures. Otherwise, reduced amounts of tubocurarine may be required
-
As with enflurane, isoflurane (Forane) relaxation of uterine muscle is not desirable if uterine contraction is required to limit blood loss.
-
Reversible reduction of GFR
-
Unlike enflurane (Ethrane), convulsive activity has not been seen with isoflurane (Forane).
-
-
 Isoflurane Advantages
Isoflurane Advantages
-
Rapid, smooth adjustment of depth of anesthesia with limited effects on pulse or respiration
-
Depth of anesthesia is easily controlled
-
No hepatic and renal toxicity
-
Cerebral blood flow and intracranial pressure are readily controlled.
-
Relaxation of skeletal muscles may be adequate for surgery
-
Arrhythmias are uncommon.
-
-
Isoflurane Disadvantage: As with halothane (Fluothane), enflurane (Ethrane), isoflurane (Forane) may cause malignant hyperthermia
-
Isoflurane Status: Isoflurane (Forane) may be among the most widely used inhalational agent.
Kennedy, S.K. and Longnecker, D.E., History and Principles of Anesthesiology In, Goodman and Gillman's The Pharmacologial Basis of Therapeutics,(Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, and Gilman, A.G.,eds) TheMcGraw-Hill Companies, Inc.,1996, p 315 - 317.
White, P. F. "Anesthesia Drug Manual", W.B. Saunders Company, 1996, p. 250.
-
-
Overview: Enflurane (Ethrane)
-
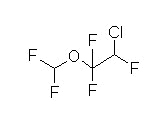
-
Halogenated, methyl ethyl ether
-
Clear, nonflammable volatile liquid (room temperature)
-
Pungent odor
-
-
Intermediate solubility + high potency leads to rapid onset/recovery (enflurane (Ethrane) alone or in combination with nitrous oxide or opioids)
-
Clinical Use: maintenance of general anesthesia
-
Systems Physiology:
-
CNS: increased ICP secondary to increased cerebral blood flow (CBF)
-
Cardiovascular:
-
Myocardial depressant
-
Decreased vascular resistance; decreased mean arterial pressure (MAP), tachycardia
-
-
Renal:
-
Renal dysfunction
-
-
Gastrointestinal:
-
Nausea
-
-
Musculoskeletal:
-
Muscle relaxation
-
-
-
 Contraindications
Contraindications
-
Renal failure
-
Long cases
-
Trigger to malignant hyperpyrexia
-
Epilepsy (may promote seizures (at concentrations > 3%); epileptiform paroxysmal spike occurrences)
-
-
Drug Interactions:
-
Potentiation: muscle relaxants
-
MAC with opioids, nitrous oxide and benzodiazepines
-
Stoelting, R.K., "Inhaled Anesthetics", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, pp 36-76
White, P. F. "Anesthesia Drug Manual", W.B. Saunders Company, 1996, p. 248.
-
 Overview: sevoflurane (Sevorane,
Ultane)
Overview: sevoflurane (Sevorane,
Ultane)
-
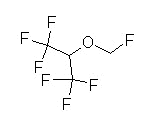
-
Fluorinated methyl isopropyl ether
-
Vapor pressure: similar to halothane (Fluothane) and isoflurane (Forane) (conventional, unheated vaporizer may be used)
-
Blood: gas partition coefficient of sevoflurane (Sevorane, Ultane) = 0.69; similar to desflurane (Suprane) allowing:
-
Rapid induction
-
Rapid recovery upon anesthetic discontinuation
-
-
Non-pungent, bronchodilation similar to isoflurane (Forane)
-
Least airway irritation among current volatile anesthetics, thereby allowing direct anesthesia induction (like halothane (Fluothane))
-
-
Metabolism: sevoflurane (Sevorane, Ultane)
-
More likely to be metabolized (3%-5% metabolized) than desflurane (Suprane)
-
Principal metabolites:
-
Inorganic fluoride (>than that seen following enflurane (Ethrane))
-
Hexafluoroisopropanol (nontoxic)
-
-
No trifluoroacetylated liver proteins formed (no anti-trifluoroacetylated liver protein antibodies)
-
Such antibodies may be seen with halothane (Fluothane), enflurane (Ethrane), isoflurane (Forane), desflurane (Suprane); these agents, metabolized to reactive acyl halide intermediates may produce:
-
Hepatoxicity
-
Cross-sensitivity between agents
-
-
-
Sevoflurane (Sevorane, Ultane) no carbon monoxide formation following exposure to carbon dioxide and absorbants
-
Level of major sevoflurane (Sevorane, Ultane) metabolite, a vinyl-ether derivative (Compound A) is significantly below possible toxic levels, even with gas flows > 1 liter per minute
-
-
 Clinical Use: sevoflurane (Sevorane,
Ultane)
Clinical Use: sevoflurane (Sevorane,
Ultane)-
First-line, excellent induction agent
-
Rapid emergence
-
Allows excellent anesthetic depth control
-
-
Systems Physiology: sevoflurane (Sevorane, Ultane)
-
CNS:
-
CNS depression (generalized)
-
Rapid emergence
-
Increased ICP
-
-
Cardiovascular:
-
Reduced vascular resistance
-
Decreased MAP (mean arterial pressure)
-
-
Pulmonary:
-
Nonirritant
-
Respiratory depression
-
-
Musculoskeletal:
-
Muscle relaxation
-
-
-
 Contraindications/Issues:
Contraindications/Issues:
-
Trigger to malignant hyperpyrexia
-
Long cases (fluoride accumulation)
-
Closed-circuit anesthesia
-
-
Drug-drug interactions
-
Muscle relaxants effect potentiation
-
MAC decreases when sevoflurane is combined with: opioids, benzodiazepines, nitrous oxide
-
Stoelting, R.K., "Inhaled Anesthetics", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, pp 36-76 White, P. F. "Anesthesia Drug Manual", W.B. Saunders Company, 1996.
DISCLAIMER
|