Anesthesia
Pharmacology: Gastrointestinal Pharmacology Practice Questions
Choose the correct answer for each question.
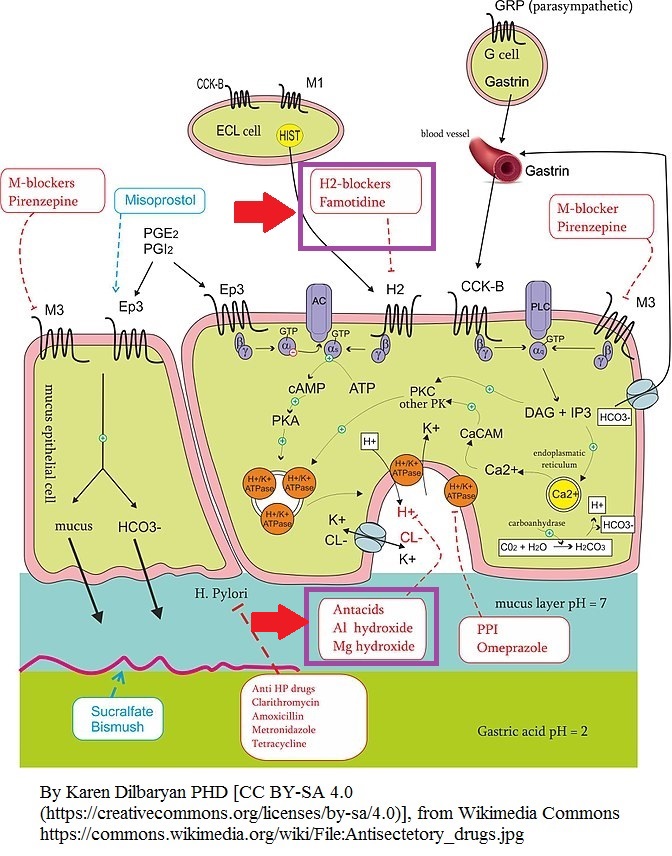
Antacids represented the primary agent for management of acid-peptic disease until:
- The development of H2 receptor blockers
- The development of proton pump inhibitors
- Both
- Neither
Use(s) of antacids in the nonprescription setting:
- Dyspepsia
- Occasional heartburn
- Both
- Neither
Antacids:
- These agents are weak bases.
- Antacids react with hydrochloric acid in the stomach forming water and a salt.
- Both
- Neither
Difference(s) between different antacid formulations:
- Dissolution rate
- Reaction rate with the acid
- Water solubility
- A & B
- B & C
- A & C
- A, B & C
Baking soda (sodium bicarbonate) reacts with hydrochloric acid (HCl) resulting in sodium chloride and carbon dioxide production.
- True
- False
Administration of sodium bicarbonate and resulting unreacted alkali:
- May cause metabolic alkalosis (at high doses)
- May cause metabolic alkalosis in renal insufficiency settings
- Both
- Neither
Sodium chloride, consequence of sodium bicarbonate interaction with HCl-clinical consequence(s):
- Fluid retention in heart failure patients
- Fluid retention in hypertensive patients
- Fluid retention in the context of renal insufficiency
- A & B
- B & C
- A & C
- A, B & C
Compared to sodium bicarbonate, calcium carbonate (Tums):
- Calcium carbonate is less soluble.
- Calcium carbonate reacts with HCL more slowly
- Both
- Neither
Sodium bicarbonate or calcium carbonate taken in high, excessive doses with calcium-containing dairy
- Metabolic alkalosis
- Renal insufficiency
- Hypercalcemia
- A & B
- B & C
- A & C
- A, B & C
Antacids may influence, through drug binding, absorption of other agents.
- True
- False