Anesthesia Pharmacology: Physics and Anesthesiology
Desflurane Vaporization Control
Desflurane vaporizer: Datex- Ohmeda Tec-6 model

30Desflurane (Suprane) requires a special vaporizer because of its unusually high volatility -- its vapor pressure is near 1 atm.
By contrast the vapor pressures of enflurane (Ethrane), isoflurane (Forane), halothane (Fluothane) are: 172 mm Hg, 240 mm Hg, and 244 mm Hg. [desflurane (Suprane): 669 mm Hg]
These values are for 20oC.
To give an idea to the consequences of such high vapor pressure, 100 ml/min passing through the vaporizing chamber will result in the development of a saturated vapor pressure equivalent to 735 ml/minute , by contrast to 29, 46, and 47 ml/min for enflurane (Ethrane), isoflurane (Forane) and halothane (Fluothane).
![]() The implication concerning the volume which
must pass through the bypass circuit (on vaporizing chamber circuit) is
that to achieve a 1% final desflurane (Suprane) vapor concentration
about 73L/minute would have to be diverted to the bypass system which is
by comparison to about 5L/minute for the other volatile agents.
The implication concerning the volume which
must pass through the bypass circuit (on vaporizing chamber circuit) is
that to achieve a 1% final desflurane (Suprane) vapor concentration
about 73L/minute would have to be diverted to the bypass system which is
by comparison to about 5L/minute for the other volatile agents.
Furthermore, because the boiling point for desflurane (Suprane) is about 22.8 oC, under some circumstances unless carefully controlled by a special vaporizer, desflurane (Suprane) could boil resulting in a completely uncontrolled delivery of anesthetic concentration.
Most variable bypass vaporizers do not require an external heat source; however this is not the case for desflurane (Suprane) vaporizers.
The MAC value for desflurane (Suprane) is significantly higher (300%-400%) compared to that those enflurane (Ethrane), isoflurane (Forane) or halothane (Fluothane).
One consequence of this relatively lower potency [PMAC1=46mmHg-55 mmHg] is that significantly more desflurane (Suprane) must be vaporized per unit time to meet the anesthetic requirements.
The vaporization process itself results in a reduction in temperature which would reduce vaporizer output; accordingly, desflurane (Suprane) vaporizers must be provided with an external source of heat.
Another consequence of the special characteristics of desflurane (Suprane) with respect to its boiling point occurs as an agent-specific vaporizer (variable-bypass) is accidentally filled with desflurane (Suprane).
An example would be filling an enflurane (Ethrane) vaporizer with desflurane (Suprane).
If the system were set up to deliver approximately 5% enflurane (Ethrane) but was mis-filled with desflurane (Suprane), the resulting desflurane (Suprane) delivery would correspond to 16 MAC (96%) at 23oC.
|
Required Output (Vapor Pressure mm Hg) |
|
Required Output (Vapor Pressure mm Hg) |
As suggested above, the special physical characteristics of desflurane (Suprane) require very carefully controlled vaporization.
To accomplish this objective the vaporizer (Tec 6) provides electrical heating and pressurization.
Although the clinical use of this vaporizer closely parallels that of other vaporizers, the internal mechanics are significantly different.
The diagram below illustrates many of the central features of the Tec 6: [Schematic of the Tec 6 desflurane (Suprane) vaporizer from reference 29 as modified from Andrews, JJ: Operating Principles of the Ohmeda Tec 6 Desflurane (Suprane) Vaporizer: A Collection of Twelve Color Illustrations.]-- reference 44.
|
|
29In the Tec 6 vaporizer, the fresh gas pathway is shown above in red and his pressure controlled by a resistor (R1 above) which establishes a working pressure.
With respect to the vaporizer components, liquid desflurane (Suprane) is contained in the sump and is held at 39oC which is considerably above its boiling point at 22.8 oC at 760 mm Hg.
Since vapor pressure is dependent on temperature, the most appropriate value for vapor pressure in the sump corresponds to about 1300 mm Hg (about 2 atm).
Note above the sump shutoff valve which will remain closed until the vaporizer reaches operating temperatures at which point the peak concentration control valve (R2) is activated to the on position.
One critical point is located at the pressure regulating valve which is located between the sump shut off valve and the concentration control valve (R2). This pressure regulating valve reduces the pressure from the vaporizing chamber to about 1.1 atm (74 mm Hg) [fresh gas flow rate equals approximately 10 L/min].
29Desflurane (Suprane) vapor output is adjusted by setting the concentration control valve.
Desflurane (Suprane) vapor coming through the vaporizing circuit mixes with fresh gas near the outlet.
As it is apparent from the diagram, the two circuits appear physically separate; however, interaction between the circuits occurs in a regulated manner.
Notes the differential pressure transducer and control electronics which influence the pressure regulating valve setting and the sump shutoff valve state.
29The purpose of the differential pressure transducer and associated electronics is to ensure equivalence in the working pressure between a fresh gas circuit and the vaporizing circuit.
For example, for a given fresh gas flow, the R1 resistor setting ensures a specific back pressure proportional to the specific fresh gas flow.
This back pressure is sensed by the differential pressure transducer which electronically alters the setting of the pressure regulating valve in a way that adjust the pressure in the vaporizing circuit to be equal to the pressure in the bypass circuit.
The differential pressure transducer would be based on a force-dependent deformable diaphragm.
Since pressure equals force x area and since the area is constant, the force will be equal (proportional) to the pressure.
One could imagine that decreasing the fresh gas flow rate would decrease the pressure on the transducer and cause a resetting of the pressure regulating valve such that the working pressure in the vaporizing circuit would equal the new reduced working pressure in the bypass circuit.
By analogy, increasing the fresh gas flow rate would increase the pressure on the differential transducer, causing an adjustment in the pressure regulating valve position resulting in an increased working pressure in the vaporizing circuit. A linear relationship exists between fresh gas flow rate in working pressure using this type of system. In this type of system then, the vaporizer output will be constant since fresh gas flow and vapor flow will be regulated in a coordinated, proportional manner.
In order to consider the desflurane (Suprane) Tec 6 vaporizer in a little more detail, we reference to figure below, adapted from reference 30 (Eisenkraft, J.B. "Anesthesia Delivery Systems", in Principles and Practice of Anesthesiology, 2nd edition, volume 1, (Longnecker, D.E., Tinker, J.H., and Morgan Jr, G.E., Mosby, St. Louis, 1998, 1001-1063.)
|
|
Again, in the figure above30, by contrast to variable-bypass vaporizers, the Tec 6 design specifically for desflurane (Suprane) diverts no fresh gas flow to the vaporizing chamber.
Note the blue dots depicting gas flowing from the machine flowmeters directly to and through a fixed resistor.
This fixed resistor referred to as Rmain above provides a resistance of about 10 cm H2O/L/min. The backpressure set by the fixed resistor in the main flow gas path sensed by the pressure transducer and in combination with control electronics adjusts the variable pressure control valve to equalize pressures.
The point is that the vapor pressure of desflurane (Suprane) is said equal to the main flow pressure and then the actual concentration desflurane (Suprane) is modified at the variable resistor stage.
30Since we will shortly considering relationships between pressure, flow and resistance, we can apply some of these concepts directly in terms of the gas pass circuitry in the Tec 6.
30For example, by definition Resistance = ![]() pressures
pressures![]() /flow.
With this principle in mind, lasts look at the main gas flow pass which
enters the fixed resistor (blue dots, towards the bottom of the above
figure). Since this is the main gas pass we would write,
Resistancemain = Pressuremain/Flowmain.
/flow.
With this principle in mind, lasts look at the main gas flow pass which
enters the fixed resistor (blue dots, towards the bottom of the above
figure). Since this is the main gas pass we would write,
Resistancemain = Pressuremain/Flowmain.
30Or, alternatively, we might write Flowmain= Pressuremain/Resistancemain . Note in the above main gas flow path, the resistor is "fixed" and is approximately 10 cm H2O/L/min (K); therefore flowmain is proportional to pressuremain.
30Now, let's look at the other pathway, the desflurane (Suprane) pathway. As before, Flowdes= Pressuredes/Resistancedes , but furthermore Concentrationdes = Flowdes / (Flowmain + Flowdes ). Assuming that Flowdes is numerically small compared to Flowmain then the earlier equation simplifies to Concentrationdes = Flowdes / (Flowmain) = (Pressuredes x K)/ Pressuremain x Resistancedes .
30The system is so designed such that Pressuredes = Pressuremain so that Concentrationdes =Flowdes /Flowmain) which would be proportional to1/Resistancedes . The relationship Concentrationdes = 1/Resistancedes is an approximation but to the extent that it is true that allows the variable resistor (which is actually the desflurane (Suprane) concentration dial) to be calibrated in terms of desflurane (Suprane) concentration. We recall the simplification which allowed us to disregard the Flowdes quantity-a simplification which is not always justifiable.
Following the sample in reference 30, we can see the application of the previous equations to a Tec 6 vaporizer gas flow problems: the intent is to set the Tec 6 in such manner as to deliver 10% desflurane (Suprane) with a gas flow of 5L/minute. Therefore:
Concentrationdes == Flowdes /(Flowmain + Flowdes ) Or
10% = 0.1 = Flowdes /(5000ml/min + Flowdes ) and by rearrangement then:
0.1 x ((5000ml/min + Flowdes )) = Flowdes
500ml/min + 0.1(Flowdes ) = Flowdes
500ml/min = 0.9 C
Flowdes = 500 ml/min/ 0.9 = 556 ml/min
Recalling that the oxygen flow rate was 5L/min in the concentration dial was set to deliver 10% agent, the vaporizer was adding 556 ml/min to the main gas flow, resulting in the following ratio 556ml/min / (5000ml/min + 556ml/min) = 10%. Also recall that the resistances, in this design between the two pressure circuits (i.e. the main flow circuits and the desflurane (Suprane) flow circuits are equal. Therefore:
Resistancedes = Pressuredes / Flowdes And
Resistancemain = Pressuremain/Flowmain And as we noted above
Flowmain / Flowdes = Resistancedes / Resistancemain = 5000ml/min / 556ml/min = 9:1
Furthermore since the resistor (fixed restrictor) was noted to be 10 cm/H2O/L/min, for a flow rate of 5000ml/min, the pressure in the main gas line would be 50 cm/H2O which would also be, for the design reasons within talking about, the pressure in the desflurane (Suprane) side of the circuit also. We already know that the desflurane (Suprane) flow rate is 556ml/min and that the equation relating resistance to pressure and flow is:
Resistancedes = Pressuredes / Flowdes = 50 cm/H2O / 0.556L/min OR 90 cm/H2O/L/min [note the unit changes implemented as required, e.g. going from a ml representation to a L representation]
30Final comments concerning desflurane (Suprane) Tec 6 vaporizers:
The reader is referred to references 29 & 30 for a more detailed description of the Tec 6 system as well as other vaporizer systems. Such detail is beyond the scope of the present consideration, the resources are highly recommended for a complete, analytical treatment.
The Tec 6 system is based in part on careful calibration during fabrication; furthermore, the calibration uses 100% O2. The accuracy of the system has been estimated to to be less than 1%, probably around 0.5% which when considered in terms of the vaporizer dial setting, the accuracy would be the setting +/- 15%.
Some additional considerations that will lead into our consideration of flow phenomenon in succeeding chapters can be introduced now. Laminar flow through a resistor, which is the "restrictor" in the above representation is described by the following relationship:
Flow = (![]() x
P x r4) /(8 x v x l); where
x
P x r4) /(8 x v x l); where ![]() =
3.142; P corresponds to the pressure differential, r = radius;
v = fluid viscosity and l = length. Qualitatively looking at
the equation for flow, one may be struck by the 4th power
dependency on radius. In hemodynamic considerations, this 4th
power dependency has significant implications. [Definition:
laminar flow -- "Streamline flow of a fluid in which the fluid
moves in layers without fluctuations or turbulence so that
successive particles passing the same point have the same
velocity. It occurs at low Reynolds numbers, i.e. low
velocities, high viscosities, low densities or small
dimensions...."45
=
3.142; P corresponds to the pressure differential, r = radius;
v = fluid viscosity and l = length. Qualitatively looking at
the equation for flow, one may be struck by the 4th power
dependency on radius. In hemodynamic considerations, this 4th
power dependency has significant implications. [Definition:
laminar flow -- "Streamline flow of a fluid in which the fluid
moves in layers without fluctuations or turbulence so that
successive particles passing the same point have the same
velocity. It occurs at low Reynolds numbers, i.e. low
velocities, high viscosities, low densities or small
dimensions...."45
Flow will be proportional to Pressure/Velocity or putting it differently Pressure will be proportional to flow x viscosity
45Definition: "viscosity A
measure of the resistance to flow that a fluid offers when it is
subjected to shear stress. For a 'Newtonian fluid', the
force, F, needed to maintain a velocity gradient dv/dx, between
adjacent planes of a fluid of area A is given by: F = ![]() A(dv/dx)
where
A(dv/dx)
where ![]() is a constant, the coefficient of viscosity. In SI units
it has the unit pascal second (in the c.g.s. system it is
measured in poise). Non-Newtonian fluids, such as clays do not
conform to this simple model"
is a constant, the coefficient of viscosity. In SI units
it has the unit pascal second (in the c.g.s. system it is
measured in poise). Non-Newtonian fluids, such as clays do not
conform to this simple model"
45Definition: "Newtonian fluid: A fluid in which the velocity gradients directly proportional to the sheer stress. It to flat plates of area A are separated d by a layer of fluid of thickness d and move relative to each other at a velocity v, then the rate of shear is v/d and the shear stress is F/A, where F is the force applied to each.
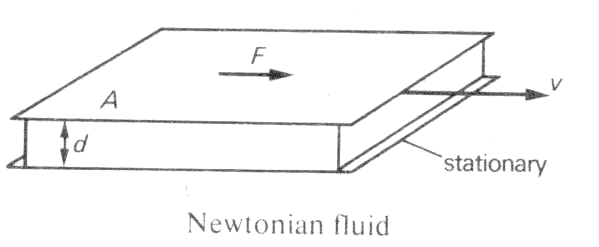 .
.
For a Newtonian fluid F/A =µv/d where µ is a constant of proportionality and is called the Newtonian viscosity. Many liquids are Newtonian fluids over a wide range of temperatures and pressures..."
30We noted in our description of the Tec 6 that flow was estimated from gas flow back pressure through the fixed resistor.
Interestingly, if the viscosity is different from the viscosity used in system calibration a new error is introduced.
For example, with decreasing gas viscosity a given flow would cause a relatively decreased back pressure which would induce a lower pressure and flow on the desflurane (Suprane) side.
This condition would lead to a flow imbalance secondary to the viscosity difference. It turns out that O2 is relatively viscous compared to gases typically used in anesthesia, whereas on the other side of the spectrum would be nitrous oxide, N2O, certainly a frequently used agent. Of course, combining O2 and N2O would be frequently done and would have a benefit of reducing the required MAC for the inhalational, volatile agent.
However, the question is what would be the impact of the reduction in gas viscosity in the Tec 6 system as a result of the O2/ N2O mixture.
As noted earlier, the direction of the effects to reduce the desflurane (Suprane) concentration secondary to the error introduced by a relatively reduced mixture viscosity.
The magnitude of the error even in the worst probable case would be about 20% of the dial setting with the worst-case set up reflected in the combination of low-flow rates and relatively higher N2O concentrations.
It is noted clinically that limited effects are observed because the reduced desflurane (Suprane) contribution to anesthesia is offset by the presence of nitrous oxide.
30Effects of hyperbaric/hypobaric conditions on desflurane (Suprane) vaporizer output:
Tec 6 vaporizers will produce, accurately, desflurane (Suprane) concentrations in terms of volume%. However, in the example given, 7% desflurane (Suprane) which is about one MAC at 760 mm Hg will correspond to a PMAC1 of about 53 mm Hg.
On the other hand, if the ambient pressure were not 760 mm Hg but 500 mm Hg, 7% desflurane (Suprane) would translate to a PMAC1 of 33 mm Hg.
Therefore from the point of view pharmacological potency, one has only achieved about two-thirds of 1 MAC.
Compensation by adjusting the vaporizer dial setting must be performed in accord with operator manual guidelines, using correction factors obtained from a graph such as the one noted above.
Citations
29Andrews, J.J. "Inhaled Anesthetic Delivery Systems" in Anesthesia 5th edition vol. 1 (Miller, R.D. editor; Cucchiara, R.F., Miller, Jr., E.D., Reves, J.G., Roizen, M.F. and Savarese, J.J., consulting editors) Churchill Livingstone, Philadelphia, 2000, pp 174-206.
30Eisenkraft, J.B. "Anesthesia Delivery Systems", in Principles and Practice of Anesthesiology, 2nd edition, volume 1, (Longnecker, D.E., Tinker, J.H., and Morgan Jr, G.E., Mosby, St. Louis, 1998, 1001-1063.
45A Concise Dictionary of Physics, Oxford University Press& Market House Books Ltd, 1992