|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiviral Drugs
Drugs Used in treating Herpes Viral Infections and related agents
IV cidofovir has been approved for use in CMV (cytomegalovirus) retinitis in AIDS patients intolerant of ganciclovir (Cytovene) or foscarnet or in those patients in which these drugs have been therapeutically ineffective.
Intravitreal cidofovir exhibits notable toxicity when used to treat CMV retinitis.
Fomivirsen (Vitavene) is an antisense oligonucleotide, FDA approved in humans in 1998 but subsequently discontinued in the U.S.5
The reason of its discontinuation in the U.S. may be related to the decline in incidence of CMV retinitis due to the highly active antiretroviral drug therapies.5
|
|
|
This agent is a phosphorothioate oligonucleotide that inhibits CMV (cytomegalovirus) replication as a result of interacting with CMV mRNA.
The phosphorothioate linkage confers resistance to nuclease degradation.
The sequences of the 21 member oliogucleotide is: 5'-GCG TTT GCT CTT CTT CTT GCG-3'
The fomivirsen oligonucleotide sequence exhibits complementarity to the CMV message transcripts of the "major intermediate early region 2" (IE2).
This region codes for proteins associated with viral gene expression and fomivirsen binding here inhibits protein synthesis and CMV replication.5
The preceding describes the "antisense" mechanism of action.
However, fomivirsen may also inhibit CMV by interfering with viral absorption to cells as well as by direct viral replication inhibition.
![]() Its unique
mechanism of action accounts for fomivirsen antiviral activity with
respect to CMV isolates resistant to nucleoside or nucleotide analogues
including ganciclovir, foscarnet, or cidofovir.
Its unique
mechanism of action accounts for fomivirsen antiviral activity with
respect to CMV isolates resistant to nucleoside or nucleotide analogues
including ganciclovir, foscarnet, or cidofovir.
Fomivirsen has been FDA approved for intravitreal administration for treatment of CMV retinitis in those patients not responding to the treatments are not being able to tolerate other treatments.
Primary toxicity is ocular inflammation such as vitreitis and iritis.
These reactions typically respond to topical glucocorticoid application.
|
|
 |
|
|
Foscarnet (Foscavir) inhibits herpes virus replication including cytomegalovirus.2
The target site for drug-mediated inhibition is DNA polymerase, at the pyrophosphate binding site.
This agent, by contrast to drugs such as acyclovir, does not require phosphorylation for activation.
Therefore, foscarnet is effective even against those herpes simplex virus (HSV) and varicella-zoster virus (VZV) isolates which are resistant to acyclovir due to low levels or absent levels of thymidine kinase.
Foscarnet is also effective against most ganciclovir-resistant cytomegalovirus (CMV) strains.
Additionally, foscarnet inhibits the HIV (human immunodeficiency virus) reverse transcriptase enzyme.2
Foscarnet has been used in management of CMV retinitis in AIDS patients and in the treatment of acyclovir-resistant mucocutaneous herpes simplex viral infections.2
![]() In CMV
retinitis patients, foscarnet administration leads to clinical
stabilization in about 90% of individuals.
In CMV
retinitis patients, foscarnet administration leads to clinical
stabilization in about 90% of individuals.
Ganciclovir or foscarnet administration appear comparably effective in treating CMV retinitis in AIDS patients; however, overall survival appeared enhanced in the foscarnet-treated group.
This improves survival result may be related to foscarnet's inherent anti-HIV effect.
![]() On the other hand, due to side effects, patients were much more
compliant in adhering to their ganciclovir treatment compared to
those receiving foscarnet.
On the other hand, due to side effects, patients were much more
compliant in adhering to their ganciclovir treatment compared to
those receiving foscarnet.
In refractory retinitis the combination of foscarnet and ganciclovir appears more effective than either agent by itself for treating refractory retinitis.
![]() Foscarnet
may be useful in other AIDS-related CMV syndromes or
transplantation-related CMV syndromes.
Foscarnet
may be useful in other AIDS-related CMV syndromes or
transplantation-related CMV syndromes.
However, foscarnet does not appear clinically effective by itself in managing CMV pneumoniae in bone marrow transplant individuals.
Foscarnet, when employed for CMV infection treatment, may also decrease the likelihood of Kaposi's sarcoma in HIV-infected individuals.
|
|
|
In the case of acyclovir-resistant mucocutaneous HSV disease, lower foscarnet doses appear accompanied by cessation of viral shedding and lead to lesion healing most of the time (75%).
For the mechanistic reasons noted earlier, foscarnet has been used to treat acyclovir-resistant HSV and VZV infections in addition to ganciclovir-resistant CMV infections.
Some CMV isolates may be resistant to foscarnet.
![]() The
major toxicity associated with foscarnet is renal dysfunction,
requiring close kidney monitoring.2
The
major toxicity associated with foscarnet is renal dysfunction,
requiring close kidney monitoring.2
Nephrotoxic effects and electrolyte disturbances may be mitigated by adequate hydration (saline) and slow drug infusion.
Evidence of nephrotoxicity include a reversible elevation in serum creatinine which may be noted in up to 50% of patients.1
Risk factors for foscarnet toxicity include:1
Higher dosages
High infusion rate
Dehydration
Pre-existing renal dysfunction and
Concurrent administration of nephrotoxic agents.
Manifestations of nephrotoxicity also include:1
Acute tubular necrosis
|
|
|
Nephrogenic diabetic insipidus
Crystalline glomerulopathy
|
|
|
|
|
Interstitial nephritis.
Pre-drug administration of saline loading may be helpful in averting or mitigating foscarnet nephrotoxicity.1
Symptomatic hypocalcemia may also accompany foscarnet administration.1
Decreases in serum Ca2+ may result in:
Paresthesia
Tetany
Arrhythmias
Seizures, as well as
Other central nervous system (CNS) abnormalities.
Concurrent administration of pentamidine appears to increase the likelihood of symptomatic hypocalcemia.1
By contrast to certain other antiviral agents, foscarnet is not notably myelosuppressive, allowing concurrent administration with myelosuppressive drugs including zidovudine (AZT).2
|
|
|
|
 |
Trifluridine, a pyrimidine nucleoside, exhibits antiviral activity against HSV-1, HSV-2, and cytomegalovirus (CMV).2
Trifluridine monophosphate is an irreversible inhibitor of thymidylate synthetase.
Trifluridine triphosphate inhibits both viral and cellular DNA polymerases, inhibiting the viral enzyme to a greater extent.
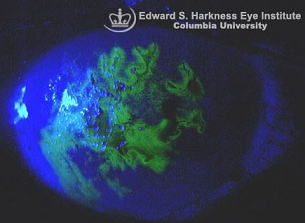
Trifluridine has been approved for treating herpes simplex viral keratitis, HSV keratitis.2
 |
|
Trifluridine appears more effective when compared to topical idoxuridine while being comparably effective to topical vidarabine in this setting.2
In certain cases topical trifluridine administration at sites of acyclovir-resistant HSV mucocutaneous infections may be helpful.2
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |