|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiviral Drugs
Drugs Used in treating Herpes Viral Infections and related agents
|
|
|
|
|
|
|
Docosanol (Abreva) inhibits fusion between the cell plasma membrane and the herpes simplex viral envelope.3
As a result viral entry into cells and consequently viral replication is prevented.
This drug, a saturated 22-carbon aliphatic alcohol, is available for topical administration, as a 10% cream.
In treating recurrent oral labial herpes, healing time is reduced by about 18 hours.3
Docosanol is not a virucidal drug in is typically not viewed as a true antiviral.4
In the 10% cream formulation docosanol (Abreva) was the first herpes simplex virus treatment sold as an over-the-counter product.4
Docosanol appears to exhibit preferential activity against lipid-enveloped viruses that use fusion mechanisms for entering target cells, compared to non-enveloped viruses.
Additionally, Docosanol does not influenced binding of herpes simplex virus to herpes simplex virus-specific target cell receptors.
Classical antiviral drugs inhibit viral DNA replication, a process allowing for HSV genomic mutations.
![]() Since
docosanol does not directly inhibit viral DNA replication, development
of resistance to docosanol is unlikely.4
Since
docosanol does not directly inhibit viral DNA replication, development
of resistance to docosanol is unlikely.4
Docosanol exhibits activity against enveloped viruses such as herpesviruses type 1 (HSV-1), herpesvirus type 2 (HSV-2) cytomegalovirus, influenzae virus, human herpesvirus-6, and respiratory syncytial virus (RSV).4
 |
 |
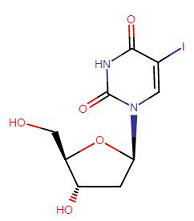
Idoxuridine (Dendrid), a viricidal drug, is an iodinated thymidine analog.1
This agent appears to inhibit replication of DNA viruses including herpesviruses and poxviruses.
Idoxuridine is not a selective agent since low concentrations inhibit the growth of both infected and uninfected cells.
Accordingly, the drug is incorporated into both viral and cellular DNA, inhibiting DNA synthesis.
DNA modified by incorporation of this analog appears more susceptible to breakage and more predisposed to transcription errors.1
![]() Resistance to the effects of idoxuridine has been noted both in
vitro and verified in viral isolates obtained from
idoxuridine-managed HSV keratitis patients.1
Resistance to the effects of idoxuridine has been noted both in
vitro and verified in viral isolates obtained from
idoxuridine-managed HSV keratitis patients.1
In the United States, idoxuridine is approved solely for topical (ophthalmic) management of HSV keratitis.1
Outside the US idoxuridine as the dimethyl sulfoxide is used for topical treatment of:
Herpes labialis
Genitalis and
Varicella-zoster.
Topical idoxuridine, in treating ocular HSV infection, is more effective in epithelial infections than in stromal infections.
Epithelial infection typically improves within a few days.5
Treatment should extend about 3 or 4 days after apparent healing.
Often ophthalmologists still prefer to denude affected corneal epithelium and do not use idoxuridine.5
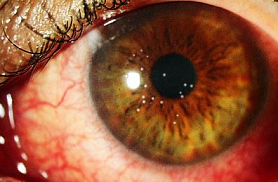
![]() Adverse
effects include itching, pain, inflammation and edema affecting the eye
or lids.1
Adverse
effects include itching, pain, inflammation and edema affecting the eye
or lids.1
A clinical study comparing acyclovir versus idoxuridine for treatment of epithelial herpes simplex keratitis was performed in the early 1980s.6
In that study 30 patients were randomized to the acyclovir group and 34 patients randomized to the idoxuridine group.6
These patients exhibited epithelial herpetic keratitis and the clinical trial evaluated these drugs for both efficacy and adverse reactions.6
Patients were treated either with 3% acyclovir ophthalmic ointment or 0.5% idoxuridine ophthalmic ointment five times a day for two weeks.
The trial results reported no difference between acyclovir and idoxuridine as antiviral agents for treating epithelial herpetic keratitis.
Factors considered included overall healing patterns, presenting conditions, duration of symptoms, prior ophthalmic steroid use and positive pretreatment herpes virus culture.
The only difference found was an increase likelihood with idoxuridine for development of an adverse reaction, superficial punctate epitheliopathy.6
 |
|
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |