|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiviral Drugs
Anti-viral drugs with activity against influenza.
Introduction:
Amantadine (Symmetrel) and an associated drug, rimantadine (Flumadine), the α-methyl derivative of rimantadine are both tricyclic amines.1
|
|
 |
 |
 |
These agents inhibit influenza A viral replication with rimantadine generally being up to 10 times more active compared to amantadine.1
Rimantadine also exhibits inhibition for a causative agent of African sleeping sickness, Trypanosoma brucei.
![]() The
efficacy of amantadine or rimantadine is dependent on possible
resistance of the influenza strain.1
The
efficacy of amantadine or rimantadine is dependent on possible
resistance of the influenza strain.1
For example, nearly all strains of influenza A H1N1 (2008-2009 influenza season) were resistant oseltamivir (neuraminidase inhibitor).
Most of the 2009 H1N1 "swine" isolate, however, were susceptible to oseltamivir but this viral isolate exhibit resistance to amantadine and rimantadine.1
|
|
|
Earlier, resistance to amantadine and rimantadine was noted in influenza A/H3N2 viruses during the 2005-2006 influenza season, also seen in the strain in 2008-2009.2
Pandemic A/H1N1 viruses in 2009-2010 also exhibit resistance to both agents.
![]() As a result, these drugs are not typically recommended for
clinical use unless the particular influenza A isolate is known
to exhibit sensitivity.2
As a result, these drugs are not typically recommended for
clinical use unless the particular influenza A isolate is known
to exhibit sensitivity.2
In that case, amantadine/rimantadine might be viewed as an option.
Amantadine and rimantadine appear effective in influenza A prophylaxis based on "large-scale" clinical studies of young adults and smaller studies involving children and the elderly.
Using these agents to prevent influenza-like disease was associated with efficacy rates of about 55-80%. Higher rates were noted when virus-specific "attack rates" were computed.2
![]() Mechanism of Action:
Amantadine and Rimantadine
Mechanism of Action:
Amantadine and Rimantadine
Amantadine and rimantadine exhibit two mechanisms accounting for their antiviral effects.
These agents inhibit an early step in viral replication most likely related to viral uncoating.1
This finding was suggested by Davies and associates who identified amantadine as an agent producing dose-related inhibition of influenza infection in three systems: tissue culture, chick embryos and mice.7
It was concluded that the agent at least in part acted by interfering with penetration of the host cell by the virus.7
The amantadine effect of inhibiting viral uncoating is shared by rimantadine.8
Other structurally-related compounds such as benzyl-substituted amantadine derivatives, also classified as viral uncoating inhibitors have also been identified.9
![]() Inhibition
of Viral Uncoating10
Inhibition
of Viral Uncoating10
As described earlier, amantadine's antiviral activity was first and generally described in 1964 (7).
Amantadine was later found to target specifically the M2 influenza A viral protein and affecting its antiviral activity at that site.
Rimantadine acts similarly.
These drugs are part of a general class, adamantanes.
Influenza A viral isolates resistant to amantadine and rimantadine which renders these drugs ineffective results from a single amino acid change in the M2 protein, given that this alteration appears to have no or limited effect on viral activity.
Newer agents (9) are in development that can block both adamantane-sensitive and-resistant isolates.
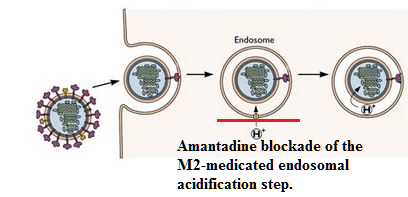
The M2 protein plays a crucial role in the release of viral ribonucleoprotein complexes into the cytoplasm.10
This process involves influenza A virus uncoating, which is defined by a fusion of viral and endosomal membranes.
Two viral proteins, M1 and M2, are important in this process.10
M1 is an important structural protein in the viral membrane and appears to link the viral membrane containing essential glycoproteins with viral ribonucleoproteins.
The M1 protein can alter virion shape.
During uncoating, M1 interactions with viral membrane and M1 interactions with viral ribonucleoproteins must be disrupted to permit complete uncoating and resultant ribonucleoprotein transport to the nucleus.
This disruption requires viral protein M2.
The M2 protein is an ion channel, the activity of which is pH dependent.
At lower pH the ion channel activity is enhanced; upon endosomal acidification, increased M2-dependent proton (H+) translocation from the endosome into the virion occurs.
This proton movement results in an acidification internal to the virus particle and this acidification (decrease in pH) promotes dissociation between M1 and viral ribonucleoproteins.
This dissociation allows for release of the viral ribonucleoprotein complexes into the cytoplasm.
 |
|
![]() Accordingly,
blockade of the M2 channel by e.g. amantadine or rimantadine is
sufficient to block this step and confers the antiviral activity
associated with these drugs.
Accordingly,
blockade of the M2 channel by e.g. amantadine or rimantadine is
sufficient to block this step and confers the antiviral activity
associated with these drugs.
|
|
|
As noted earlier however, a mutation, even a single mutation, in the M2 channel protein may be sufficient to confer resistance of the virus to the adamantane effects.10
These agents also affect a late step in viral assembly, related to hemagglutinin processing.1
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |