|
|
|
Medical Pharmacology Chapter 36: Antiviral Drugs
Antiviral Drugs
More about Influenza Viruses.
Orthomyxoviruses and paramyxoviruses are the two families originally described by "myxoviruses".4 (continued)
![]() Nucleocapsid
release from the virion: the role of the M2 protein
Nucleocapsid
release from the virion: the role of the M2 protein
|
|
|
|
|
|
The M2 protein is small with an N-terminal external domain, along with a single transmembrane domain with a larger internal domain.4
The essential feature of the M2 protein resides in its ability to form a highly selective transmembrane ion channel that allows protons (H+) to partition into the membrane.
Structurally, the M2 protein creates a pore utilizing 4 parallel transmembrane α-helices (tetrameric) which structurally defines the viral envelope pore.
|
|
|
An important step in virus replication is release of the viral nucleocapsid from the virion and it is this process that is made easier by the M2 H+ channel.
The consequence of this channel facilitating proton translocation to the interior of the virion causes endosomal acidification.4
As a result of this acidification (higher [H+]), the affinity of the matrix protein M1 for the nucleocapsid is reduced, thus statistically increasing the likelihood of nucleocapsid release into the cytoplasm, following membrane fusion.
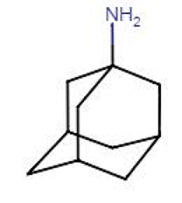
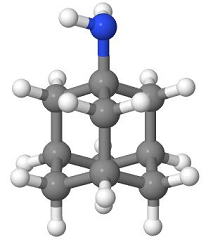
![]() The
anti-influenza viral drug amantadine is a specific inhibitor at the
M2 membrane H+ channel.
The
anti-influenza viral drug amantadine is a specific inhibitor at the
M2 membrane H+ channel.
Therefore, with amantadine-mediated channel blockade, viral nucleocapsid release is diminished or incomplete, thus blocking infection.4
 |
 |
Orthomyxoviruses (influenza virus) replicate in the host cell nucleus, by contrast to most other RNA viruses.4
1. Virion genomes are initially transported to the nucleus for transcription.
2. Viral mRNAs then move to the cytoplasm for translation into protein.
3. Viral proteins then move back to the nucleus for the purpose of regulating genomic replication.
4. Nucleocapsids are formed using the newly replicated viral genome with the nucleocapsids then moving back to the cytoplasm, allowing virion assembly at the host cell plasma membrane.
5. Nucleocapsids move from the cytoplasm to the nucleus through a nuclear membrane pore.
The NP protein, nucleocapsid protein, as well as polymerase proteins containing nuclear localization recognition capabilities, mediating interaction with cellular importin-α.
The resulting complex then interacts with importin-β.
This assembly docks with nuclear pore complexes and facilitates translocation of proteins across the nuclear membrane important for replication .
Copying of viral negative-sense RNA genome segments, described earlier, is then initiated utilizing cellular RNAs as transcription primers.4
|
|
|
|
Primers for synthesis of viral mRNAs are "capped" 5' ends of cellular pre-messenger RNAs.4
Capping represents an an altered nucleotide on the 5'-end of, in this case precursor messenger RNA.
The capping process, which occurs only in the nucleus, is important in the creation of both stable and mature mRNA.
The 5' cap (on the 5' mRNA end) is composed of a guanine nucleotide linked to the mRNA by means of a 5' to 5' triphosphate bridge.
|
|
Capped viral mRNAs are derived from these host-cell cellular pre-mRNAs that had been used to prime transcription.
This is the mechanism by which orthomyxoviruses (e.g. influenza virus) makes capped messenger RNAs even without its own coding for capping enzyme.
Normally, cellular pre-messenger RNAs are exported to the cytoplasm where they provide a pool of cellular pre-messenger RNA, thus supporting influenza virus mRNA production.
If this pool is not actively replenished by an intact cellular RNA synthesis system, the cellular pre-messenger RNA pool needed by the virus for its mRNA synthesis would become rapidly depleted.4
![]() Thus,
for influenza virus, given the need for cellular
RNA synthesis, agents that interfere with
cellular RNA synthesis inhibit influenza viral
replication.
Thus,
for influenza virus, given the need for cellular
RNA synthesis, agents that interfere with
cellular RNA synthesis inhibit influenza viral
replication.
Examples of such agents include:
actinomycin-D
|
|
|
α-amanitin.4
|
|
|
The reader is directed to reference 4 for detailed discussion of additional, detailed steps in influenza A viral replication and regulation.4
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |