|
|
|
Autonomic Pharmacology--Adrenergic Drugs
|
|
|
|
Epinephrine
Adrenal Medulla:53
Epinephrine is one of several adrenal medullary catecholamines; other important vasoactive substances secreted by the adrenal medulla include norepinephrine and dopamine.
In humans, catecholamine output measured in the adrenal vein consists mainly of epinephrine.
The source for circulatory norepinephrine is norepinephrine released by adrenergic, sympathetic nerve terminals.
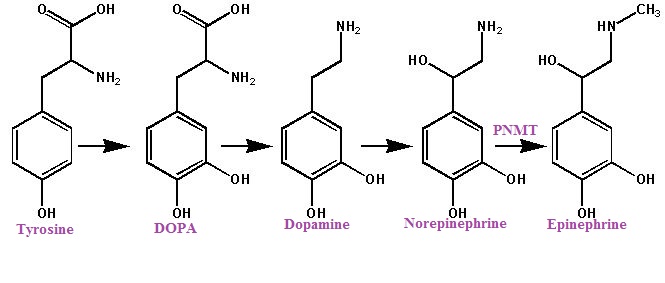
As noted earlier, hydroxylation and decarboxylation of tyrosine results in norepinephrine formation and an additional step, norepinephrine methylation, results in epinephrine. This last step is catalyzed by phenylethanolamine-N-methyltransferase (PNMT), an enzyme found prominently in the adrenal medulla and the brain.
 |
Adrenal medullary PNMT is regulated in part by glucocorticoids, which induce higher enzyme concentrations. Normal adrenal medullary development requires glucocorticoids; note that with 21β-hydroxylase deficiency which results in reduced fetal glucocorticoid secretion, the adrenal medulla is dysplastic. Furthermore, if untreated, 21β-hydroxylase deficiency results in low postnatal circulating catecholamine.
|
About 70% of plasma epinephrine is conjugated to sulfate. The sulfate conjugates are biologically inactive. Epinephrine is primarily synthesized in the adrenal medulla and epinephrine localized elsewhere appears most likely due to absorption from the blood rather than local synthesis. However, following bilateral adrenalectomy, low epinephrine levels in the circulation appear; the source of this epinephrine, possibly cardiac adrenergic cells, has not been definitively determined.
Adrenal medullary epinephrine is stored in granules along with ATP and chromagranin A.
Epinephrine secretion occurs following preganglionic neuronal acetylcholine release; these neurons innervate the secretory cells. Acetylcholine increases Ca2+ channel conductance thus allowing increased intracellular Ca2+. Ca2+ influx is an important event in promoting granular exocytosis.
Adrenal medullary epinephrine-containing cells also secrete opioid peptides with most of the circulating met-enkephalin (the precursor molecule is preproenkephalin) derived from the adrenal medulla.
Lastly, adrenomedullin, a polypeptide that promotes vasodepressor responses is localized in the adrenal medulla.
Epinephrine: Target Organ Effects
Cardiovascular effects of epinephrine are represented in the table below. These effects take into account both activation of β- and α- receptors as appropriate. Epinephrine effects are compared to those of norepinephrine, both following IV administration.
|
Cardiac Effects |
Epinephrine | Norepinephrine |
|
Heart Rate |
|
|
|
Stroke Volume |
|
|
|
Cardiac Output |
|
no change,
|
|
Coronary Blood Flow |
|
|
|
Arrhythmias |
|
|
|
|
Blood Pressure Effects |
Epinephrine |
Norepinephrine |
|
Systolic Arterial pressure |
|
|
|
Mean Arterial |
|
|
|
Diastolic Arterial pressure |
|
|
|
Mean Pulmonary Pressure |
|
|
|
Epinephrine is a potent vasopressor
Systolic pressure increases to a greater extent than diastolic (diastolic pressure may decrease)
pulse pressure widens
Epinephrine increases blood pressure by:
![]() enhancing cardiac
contractility (positive
inotropic effect):
β1-receptor
effects
enhancing cardiac
contractility (positive
inotropic effect):
β1-receptor
effects
![]() increasing
heart rate (positive
chronotropic effect):
β1-receptor
effects.
increasing
heart rate (positive
chronotropic effect):
β1-receptor
effects.
vasoconstriction α1 receptor effects
precapillary resistance vessels of the skin, kidney, and mucosa
veins
If epinphrine is administered relatively rapidly, the elevation of systolic pressure is likely to activate the baroreceptor system resulting in a reflex-mediated decrease in heart rate.
At lower epinephrine doses a reduced effect on systolic pressure is observed.
Diastolic pressures may decrease as peripheral resistance is reduced. A decrease in peripheral resistance is a result of ß2-adrenergic receptor activation.
|
|
|
Peripheral Circulation Effects |
Epinephrine |
Norepinephrine |
|
Total Peripheral Resistance |
|
|
|
Cerebral Blood Flow |
|
no change,
|
|
Muscle Blood Flow |
|
no change,
|
|
Cutaneous Blood Flow |
|
|
|
Renal Blood Flow |
|
|
| Splanchnic Blood Flow |
|
no change,
|
|
Epinephrine exerts a number of cardiac stimulatory effects.
Epinephrine is a primary agonist at β1 adrenergic receptors influencing both myocardial and specialized conducting tissue and pacemaker cells. α- and other β-adrenergic receptor subtypes are also present.
Epinephrine administration results in an increase in heart rate and changes in cardiac rhythm.
Increased myocardial work and associated oxygen consumption are increased in accord with enhanced contractility with shortened cardiac systole.
Myocardial mechanical changes subsequent to epinephrine include:
increased rate of rise of isometric tension
increased relaxation rate
reduced time to peak tension
increased excitability (which may predispose to arrhythmias)
increased rate of pacemaker cell activity (i.e., increased slope of phase 4 depolarization)
increased likelihood of automaticity in some cardiac regions
and increases contractile force.
Epinephrine-induced positive chronotropism (increase in rate) is associated mainly with reduced systole with preservation of diastolic duration.
Duration of diastole is important in ensuring sufficient myocardial filling time.
With epinephrine, the pacemaker cells of the sinoatrial (SA) node reach threshold potential more quickly; this effect occurs because of an increased slope of membrane depolarization towards threshold (phase 4 depolarization).
|
|
 |
Action potential amplitude and maximal depolarization rate (phase 0) are also increased. Increased automaticity associated with epinephrine may lead to changes in which SA nodal cells pace the heart, as "latent" pacemakers may emerge.4
Cells which exhibit spontaneous resting depolarization may be latent pacemakers; however, normally, SA nodal cells reach threshold first.
Since epinephrine will accelerate diastolic depolarization in Purkinje fibers, pacemaker activity in these fibers is enhanced. Phase 4 (diastolic) membrane potentials tend to be stable in normal atrial and ventricular muscle fibers with epinephrine usually exhibiting minimal effect.
However, at high epinephrine doses, premature ventricular contractions may occur suggesting the possibility that more serious arrhythmias may ensue.
If the myocardium has been "sensitized", an effect which has been associated with prior administration of some anesthetics, circulating catecholamines may be sufficient to induce serious ventricular arrhythmias ranging from extrasystoles to fibrillation.
In some circumstances conduction velocities through the Purkinje system may be low, secondary to reduced membrane potentials (more positive).
Conduction velocity is closely coupled to the value of the membrane potential of the time of depolarization.
With reduced membrane potentials (more positive), fewer inward depolarizing channels are activated synchronously; therefore, both the magnitude of the depolarizing effect is less and the rate of rise is reduced.
Less inward current flowing translates directly to reduced rate of action potential propagation.
Epinephrine tends to increase the membrane potential in Purkinje cells and mitigate or reverse slow action potential propagation due to pre-existing reduced membrane potentials.
Epinephrine decreases the human atrial ventricular (AV nodal) refractory period; however, if the dosage of epinephrine is sufficient to induce a compensatory, vagal (parasympathetic) response, an opposing effect at the AV node would occur (a lengthening of the refractive period).4
This reflex response is another example of the compensatory action inherent in the autonomic nervous system. In this example, an increase in blood pressure due to epinephrine results in an increase in vagal tone, which not only reduces heart rate and contractility but also acts in opposition to the epinephrine effect on the AV nodal refractory period.
The interplay between sympathetic and parasympathetic tone may predispose to ventricular arrhythmias in view of the epinephrine proarrhythmic action being mitigated in part by blockade of reflex vagal responses.
Epinephrine-induced arrhythmias are likely due to increases in cardiac automaticity. β-adrenergic receptor antagonists such as propranolol (Inderal) exerts a protective effect against epinephrine-induced arrhythmias.
Accidental intravenous epinephrine administration (intended subcutaneous injection) can result in PVCs (premature ventricular contractions) as well as more serious arrhythmias. Epinephrine administration can induce changes in the ECG, particularly T-wave changes.
Reduced T-wave amplitude and ST segment changes may be observed. ST segmental changes seen following epinephrine appear similar to those observed in angina pectoris.
|
|
| This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |