|
|
|
Medical Pharmacology Chapter 13 Opioids: Advanced Topics
Review
1History: The idea or least popularization of "balanced anesthesia" along with the introduction of IV, short acting thiobarbituates lead to renewed interest in opioids as anesthetic drugs.
Previously morphine, for example in the 1850s was used as a supplement during chloroform anesthesia and then postoperatively to manage pain.
In terms of complete anesthesia, relatively high morphine concentrations (1-2 mg/kg) + scopolamine (1-3 mg/70 kg) was administered (divided doses) as a complete anesthetic.
Perioperative morbidity resulted in this technique being essentially rejected and along with opioids were uncommonly used intraoperatively for about three to four decades.
As noted, availability of thiobarbituates along with the idea of balanced anesthesia provided the framework for contemporary opioid use intraoperatively.
1An important step was the synthesis of meperidine and then it subsequent use in combination with nitrous oxide (+/- D-tubocurarine).
Different approaches to the use of nitrous-narcotic or balanced anesthesia evolved beginning with thiopental for induction and maintenance along with D-tubocurarine and opioids including morphine and meperidine.
Later, various IV supplementation was used involving hypnotics, sedatives, other analgesics and tranquilizers, particularly following the introduction of Innovar-nitrous oxide anesthesia.
Innovar is the combination of droperidol and fentanyl.
The use of many agents contributed to the contemporary definition of "balanced anesthesia", with the rationale that each pharmacological element addressed the particular need -- needs could include unconsciousness, amnesia, muscle relaxation, analgesic, blunting of the autonomic responses.
1With the introduction of potent inhaled agents, opioids as well has various sedative-hypnotics (e.g. midazolam) collectively defined (and defined) contemporary combinations.
The use of opioids and benzodiazepines had in advantage of reducing the amount of potent inhaled agent required.
Reduced amounts of inhalational anesthetics have the benefit over reduced cardiovascular depression and facilitated anesthesia recovery.
1Use of large opioid doses as the primary or sole anesthetic was an approach reintroduced by Lowenstein et al.1a and Stanley and Webster.1b
In patients without cardiac disease, administration of 100% oxygen with IV morphine (0.5-3 .0 mg/kg) did not adversely affect the cardiovascular system; moreover, cardiac performance was actually improved by this formulation in some patients with valvular heart dysfunction.
These early studies1a,1b stimulated additional work in the field of high-opioid anesthesia especially evaluating this approach for patients with limited cardiovascular reserve but needing to undergo significant operations1c,1d,1e.
Difficulties with high-dose opioid anesthesia resulted in a reduction of morphine utilization as the sole anesthetic.
These problems included incomplete or inadequate amnesia, histamine release, significant and prolonged respiratory depression, prominent venovasodilatation causing increased blood volume requirements, hypotension, and hypertension.
Many of these adverse hemodynamic consequences following high-dose morphine use was not observed with high-dose fentanyl.
In contemporary balanced anesthesia applications, fentanyl is an important component when combined with inhaled agents and can also be used at higher doses as a primary or even sole anesthetic.
In this case, however, respiratory depression may be prominent and prolonged such that tracheal extubation might be delayed solely for this reason.
Agents other than fentanyl and belonging to the same category of high-potency opioids have been introduced, including sufentanil, alfentanil and remifentanil.
Some other properties are summarized below:
Relative to fentanyl, sufentanil is more potent and possibly more effective when used as a primary or sole agent.
Alfentanil exhibits very rapid onset and might be more predictable in determining the dose required to obtain adequate plasma drug levels and to blunt surgical stimuli responses when the agent is administered with nitrous oxide.
Remifentanil demonstrates very rapid onset of action but exhibits extremely short duration of action -- not context dependent elimination, e.g. short duration of action doesn't depend on length of time of drug administration.
Rapid resolution of significant opioid activity at the end of surgery is an important feature of this agent.
![]() One of the major pharmacokinetic problems associated
with opioid administration is predictability of plasma
concentration.
One of the major pharmacokinetic problems associated
with opioid administration is predictability of plasma
concentration.
A standard dose can results in significant plasma concentration variability (five-fold variability).
Accordingly therapeutic plasma levels very also (3-5 fold).
Plasma sufentanil and alfentanil concentrations are reduced more rapidly compared to fentanyl resulting in a more rapid patient recovery following termination of IV infusions.
|
|
|
|
morphine |
meperidine |
|
|
|
|
fentanyl |
sufentanil |
|
|
|
Opioid Structural Classifications
A nonchemical classification system can describe opioids as naturally occurring, synthetic or semisynthetic.
From the point of view of clinical significance, the only naturally occurring opioids, obtained from the poppy plant (Papaver somniferum) are morphine, codeine, and papaverine.
Papaverine is a smooth muscle relaxant but does not exhibit opiate activity.
|
|
1Naturally occurring opioids are divided into two chemical classes: (1) five-ring structures, phenanthrenes represented by morphine and codeine and (2) three-ring structures represented by papaverine.
1Semisynthetic agents are derivatives of morphine and include codeine, heroin (diacetylmorphine ), and thebaine which is an inactive opium derivative but a precursor of several compounds used clinically, such as oxymorphone, oxycodone, and naloxone.
The thebaine derivative etorphine can be used in immobilization and wildlife anesthesia given that it is three orders of magnitude more potent than morphine.
|
|
|
|
oxymorphone |
oxycodone |
|
|
|
|
heroin |
naloxone |
1Synthetic opioid compounds fall into one of four categories:
Morphinan derivatives e.g. levorphanol:
basic morphinan structure = 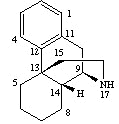
Diphenyl or methadone derivatives (methadone,d-propoxyphene)
Benzomorphan compounds e.g. pentazocine
Phenylpiperidine derivatives such as meperidine,
fentanyl, alfentanil, remifentanil, and sufentanil. Basic
phenylpiperidine structure =![]()
The major role in contemporary anesthesia with respect opioid use is filled by phenylpiperidine derivatives.
![]() 1Opioid
functional classifications include (a) agonist, (b) partial agonist, (c)
agonist-antagonist and (d) antagonist.
1Opioid
functional classifications include (a) agonist, (b) partial agonist, (c)
agonist-antagonist and (d) antagonist.
Interaction of an opioid receptor with an agonist results in a physiological effect; furthermore, an agonist may exhibit a maximal effect with respect to some endpoint, such as analgesia whereas a partial agonist while also mediating its effect through opioid receptor interactions results in some reduced level of analgesia.
1Notice the difference between agonist and partial agonist maximal effects (Fig 10-2, ref. 1)
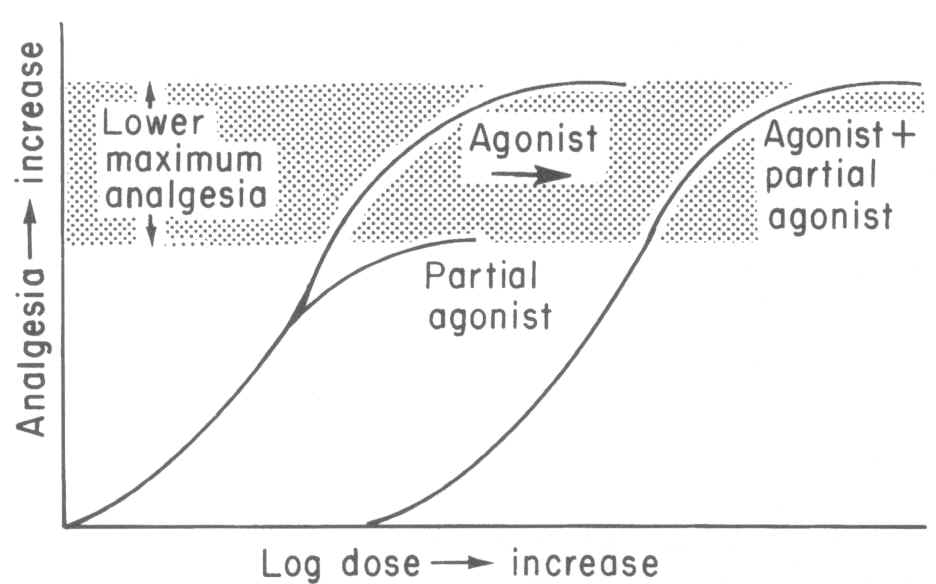
An example of partial agonist would be buprenorphine, which
by definition, cannot exert the same magnitude of opioid effect (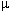 -mediated)
when compared to a full agonist.
-mediated)
when compared to a full agonist.
Nalbuphine is an example of a mixed
agonist-antagonist agent, acting as an agonist at one receptor type (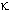 ) and as a result promoting a weak spinal-cord mediated analgesia on one
hand and an antagonist action and the second receptor type (
) and as a result promoting a weak spinal-cord mediated analgesia on one
hand and an antagonist action and the second receptor type (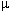 )
which manifests as reversal of agonist-mediated respiratory depression.
)
which manifests as reversal of agonist-mediated respiratory depression.
Structure-Activity Relationships
1Three-dimensional structures of opioids reflect their complexity.
Furthermore, opioids may exist as two optically active isomer forms, with the l form (optical rotation to the left) being the form producing analgesia.
Analgesic activity is closely associated with stereochemical structure with minimal molecular changes, even ionization state changes, sufficient to cause substantive alteration in pharmacological activity.
Using morphine as the prototypical structure we can identify the fundamental phenylpiperidine form which is an aromatic ring attached to a six-membered ring which contains one nitrogen and five carbons.
This basic structure is found in other opioids such as fentanyl.
The pentacyclic structure approximates a "T" shape, shown below.
|
|
|
|
1Bailey, PL, Egan, TD, Stanley, TH, "Intravenous Opioid Anesthetics", in Anesthesia 5th edition, Miller, R.D., editor, Churchill Livingstone, Philadelphia, 2000, 273-377 (references secondarily sourced from this primary reference our noted below in the indented references)
1aLowenstein, E, Hallowell P, Levine FH et al.: Cardiovascular response to large doses of intravenous morphine in man.N. Engl J Med 281:13 89, 1969
1b Stanley, TH, Webster LR; Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth Analg 51:901, 1972.
1c Arens, JR, Benbow, BP, Ochsner JL et al: Morphine anesthesia for the aorto-coronary bypass procedures. Anesth Analg 57: 411, 1978
1d Stanley, TH, Gray NJ, Staford W. et al: The effects of high-dose morphine on fluid and blood requirements in open-heart operations. Anesthesiology 38:536, 1973.
1e Stoelting, RK, Gibbs PS, Creasser CW et al: Hemodynamic in ventilatory response to fentanyl, fentanyl-droperidol, and nitrous oxide in patients with acquired valvular heart disease. Anesthesiology 42:319, 1975.
Coda, BA, "Opioids" in Clinical Anesthesia, 4th edition, Barash, PG, Cullen, BF, Stoelting, RK, editors, Lipincott Williams and Wilkins, Philadelphia, 2001, 345-375
Ross, AF, Gomez, MK, Tinker, JH "Anesthesia for Adult Cardiac Procedures in Principles and Practice of Anesthesiology, 2nd edition, Longnecker, DE, Tinker, JH, Morgan, GE, eds, Mosby, St. Louis, 1998, pp. 1659-1698.
Heish, JC, Carr, DB "Choosing a Therapeutic Approach: Opioids" in The Massachusetts General Hospital Handbook of Pain Management (Borsook D, LeBel, AA, McPeek, B, eds) Little, Brown and Company, Boston, 1996, pp 47-75.
DISCLAIMER
|