|
|
|
1Intravenous Opioid Anesthetics: Pruitus
The occurrence of opioid-induced pruritus is well established; however, the mechanism remains to be elucidated. Histamine release does not appear to be the cause, given that certain opioids that do not promote histamine release still cause pruritus. Allergic reactions in the presence of drug solution preservatives also do not account for this phenomenon.
1Opioids: Some Therapeutic Actions
1Ventilation: One consequence of pain/anxiety may be respiratory alkalosis secondary to increased spontaneous ventilation. Hyperventilation can also follow from some brainstem disorders. Management of hyperventilation is provided by opioids by means of reducing both pain and central ventilatory drive. Furthermore, postoperative respiratory dysfunction can follow from inadequate pain relief. Overall, opioid analgesic treatment can enhance synchronous breathing with a reduction in voluntary muscle tone, ultimately improving dynamic total respiratory compliance in awake but mechanically ventilated patients in the ICU setting. Of course, the caveat is that large doses of opioids can reduce pulmonary compliance (increased rigidity) as well as in directly impair ventilation.
1Anti-cough: (antitussive)-- Central mechanisms are responsible for opioid-induced antitussive effects.
In about half of patients receiving IV bolus fentanyl,
sufentanil and alfentanil can initiate brief coughing episodes,
possibly due to a pulmonary chemoreflex mediated by C-fiber receptors.
This cough reflex following opioid injection is not affected by atropine
pretreatment, though suggesting a mechanism other than a vagal
one. Opioid-induced cough could occur by stimulation of
"Ericsson" receptors associated with tracheal smooth
muscle. Finally, pretreatment using inhalational  -
adrenergic agonists decreases the likelihood of IV opioid
injection-induced cough.
-
adrenergic agonists decreases the likelihood of IV opioid
injection-induced cough.
1Suppression of upper airway, tracheal & lower respiratory tract reflexes: This type of reflex suppression although well accepted is not associated with and elucidated mechanism.
1Opioid-induced suppression of somatic and autonomic reactions associated with endotracheal intubation: opioid administration permits endotracheal intubation without "bucking" or coughing, syndromes that can lead to hypoxemia, hemodynamic instability, hypercarbia and other pathophysiological conditions. Reduced lung volume is associated with bucking and this reduced lung volume along with disturbances in ventilatory pattern can result in nonphysiological gas exchange. Coughing and bucking on emergence might be expected in those patients who received a potent inhalational agent but did not receive opioids. Utilization of opioids, that is proper utilization, can lead to patient awakening without such problems even if the endotracheal tube remains in place.
1Bronchomotor tone: opioids may prevent increased bronchomotor tone. These agents also are helpful in managing dyspnea and other respiratory manifestations associated with asthma and congestive heart failure. Fentanyl, which exhibits antihistaminergic, antiserotonergic, as well as antimuscarinic effects may be more effective than morphine for managing patients with asthma or bronchospastic disorders. Lastly, suctioning-induced tracheobronchial stimulation can induce a pulmonary vasoconstrictive response which may be prevented or reduced by opioids.
1Opioids: Adverse Effects & Non-Therapeutic Effects
1One of the prominent effects of opioids is respiratory depression. Opioid receptors have been found at high concentration in the number of sites in the brain, including the nucleus solitarius, nucleus retroambigualis, and nucleus ambiguus. Opioid induced respiratory effects are mediated by specific chemosensitive brain regions. Opioids also prevent normal physiological function in pontine and medullary respiratory centers.
(The Digital Slice of Life is a cooperative project with the Slice of Life office, KUED Media Solutions, and the Knowledge Weavers Project.) http://medlib.med.utah.edu/kw/
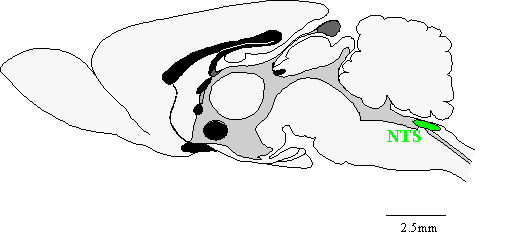 |
Attribution: Dr. Al Ferguson, Queen's University at Kingston
1![]() -opioid
receptors are stimulated by opioids, resulting dose-dependent respiratory
depression by means of direct action on brain-stem respiratory
centers. CO2 stimulatory effects on ventilation are
diminished by opioids and in accordance with this finding, ventilatory slope
and occlusion pressure responses to CO2 are diminished with
minute ventilatory responses to increases in PaCO2 exhibit curve shifting to the right. The apneic threshold and resting end-tidal PCO2
are increased by opioids
-opioid
receptors are stimulated by opioids, resulting dose-dependent respiratory
depression by means of direct action on brain-stem respiratory
centers. CO2 stimulatory effects on ventilation are
diminished by opioids and in accordance with this finding, ventilatory slope
and occlusion pressure responses to CO2 are diminished with
minute ventilatory responses to increases in PaCO2 exhibit curve shifting to the right. The apneic threshold and resting end-tidal PCO2
are increased by opioids
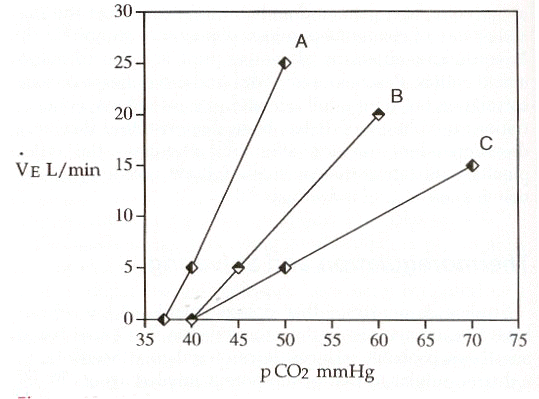 |
"In this illustration, the slope of line A is approximately 2 L/min/mmHg, although wide range of responses in humans exists. The slope of the ventilatory response to CO2 is reduced to 1 and 0.5 and lines B and C respectively. Lines B and C are also shifted to the right of line A. Line speed and see have the same intercept along the X axis, indicating no displacement or shift of line C compared with line B." (Reference 1)
1Hypoxic ventilatory drive is attenuated by the opioids with carotid body chemoreception and hypoxic drive also blunted or eliminated even by low, analgesic opioid doses. The increased respiratory drive associated with increasing airway resistance is also reduced by opioids. The dose-dependent reduction in spontaneous respiratory rate allows opioid titration to effect in the anesthetized patient. On the other hand, the extent of opioid-induced respiratory depression cannot be reliably judged by only observing respiratory rate, particularly in the postoperative environment. Elimination of spontaneous respiration by the opioids is not necessarily associated with loss of consciousness; therefore, individuals receiving high-dose opioids may be responsive to verbal commands even those which request that they breathe.
1Opioid-induced influences on respiratory rhythm and pattern include the following changes:
irregular and/or periodic breathing
expiratory delay
respiratory pausing
alteration in tidal volume (decreased or increased)
1Onset of respiratory depression following opioid administration:
Peak onset of respiratory depression following morphine (analgesic dose level) is slower than that observed following comparable fentanyl doses (the proportionality is about 30 minutes versus about seven minutes). Explanation for this difference references the lower lipid solubility associated with morphine -- this lower lipid solubility is responsible for morphine plasma concentrations in onset of action being nearly the same following IV versus IM administration. In the immediate postoperative timeframe, the use of morphine as an IV analgesic is therefore perhaps not the best choice.
Duration of respiratory depression -- small doses of morphine induce a
respiratory depression that typically lasts longer than that observed
following an equipotent fentanyl dose. Another comparison involves IV
fentanyl versus meperidine -- in that case IV fentanyl (100 & 200 ![]() g/70kg)
administration induces respiratory depression that does not last as long
as and equipotent meperidine dose (65-75 mg/70kg). In the same
study, faster onset in peak effect following fentanyl was noted,
compared to meperidine. The shorter onset include recovery
associate with fentanyl compared to morphine and meperidine does not
obviate the observation that small doses (2
g/70kg)
administration induces respiratory depression that does not last as long
as and equipotent meperidine dose (65-75 mg/70kg). In the same
study, faster onset in peak effect following fentanyl was noted,
compared to meperidine. The shorter onset include recovery
associate with fentanyl compared to morphine and meperidine does not
obviate the observation that small doses (2![]() g/kg)
induces respiratory depression that lasts for a rather longtime (>
one hour). By contrast, sufentanil (0.1-0.4
g/kg)
induces respiratory depression that lasts for a rather longtime (>
one hour). By contrast, sufentanil (0.1-0.4 ![]() g/kg)
administration results in shorter-lasting respiratory depression but
longer-lasting analgesia compared to fentanyl (1-4
g/kg)
administration results in shorter-lasting respiratory depression but
longer-lasting analgesia compared to fentanyl (1-4 ![]() g/kg).
g/kg).
Initial postoperative respiratory depression is typically not
observed when fentanyl (4-8 ![]() g/kg)
is administered during induction; however, there's a possibility of
significant respiratory depression noted five or more hours later.
With higher doses of fentanyl (50-100
g/kg)
is administered during induction; however, there's a possibility of
significant respiratory depression noted five or more hours later.
With higher doses of fentanyl (50-100![]() g/kg)
persistent respiratory depression may require ventilatory support for
6-18 hours. Moreover, with intermediate dose levels (20-50
g/kg)
persistent respiratory depression may require ventilatory support for
6-18 hours. Moreover, with intermediate dose levels (20-50 ![]() g/kg)
the possibility of the need of postoperative mechanical ventilation
should be considered.
g/kg)
the possibility of the need of postoperative mechanical ventilation
should be considered.
1Recovery of respiratory function
Alfentanil and sufentanil are associated with a more rapid recovery of respiratory function compared to fentanyl -- at least with fentanyl, a possible explanation at the molecular level is the more rapid dissociation of alfentanil from the receptor compared to other agents. This more rapid dissociation has been interpreted to possibly explain a decrease likelihood of respiratory depression.
With remifentanil, independent of dosage, and a residual effects dissipate extremely rapidly (5-15 minutes) following discontinuation of administration.
1Factors that Influence Opioid-Induced Respiratory Depression
1Duration and magnitude of respiratory depression following opioid administration can be influenced by a number of factors.
Factors that increase magnitude and/or duration of opioid-induced respiratory depression include:
![]() dose
dose
Intermittent bolus (by contrast to continuous infusion)
![]() Brain
penetration/drug delivery
Brain
penetration/drug delivery
![]() Distribution
(
Distribution
(![]() cardiac output)
cardiac output)
![]() Un-ionized
fraction (respiratory alkalosis)
Un-ionized
fraction (respiratory alkalosis)
![]() Reuptake
from the brain (interoperative respiratory alkalosis)
Reuptake
from the brain (interoperative respiratory alkalosis)
![]() Clearance
(
Clearance
(![]() hepatic
blood flow for example inter-abdominal surgery)
hepatic
blood flow for example inter-abdominal surgery)
Secondary peaks in plasma opioid concentrations (reuptake of opioid from muscle, lung, fat, intestine reservoir)
![]() Ionized
opioid receptor site (postoperative respiratory acidosis)
Ionized
opioid receptor site (postoperative respiratory acidosis)
Sleep
![]() Age
Age
Metabolic alkalosis
1Sleep: Opioids potentiate the normal right shift of the PaCO2-alveolar ventilation curve, observed during non-REM sleep. Respiratory, cognitive, and hemodynamic anomalies are probably responsible for the hypoxemia associated with sleep postoperative (several days). Natural sleep as well as morphine reduce the thoracic component of breathing with limited effect on the diaphragmatic component. Tonic and phasic upper airway muscle activity associated with breathing is also impair by sleep; this effect may be problematic one patients have an opioid-based anesthetic in the context of surgery which is associated with limited postoperative pain. In this eventuality, seemingly normal breathing while awake can transition to inadequate breathing during sleep. For those patients with impaired abdominal breathing or problems with their airway function (e.g.in significantly obese patients or in patients who exhibit sleep apnea), opioid analgesics may increase the probability of adverse respiratory events.
1Age: Anesthetic and respiratory depressant effects of opioids are more apparent in older patients. Furthermore, older patients exhibit a higher opioid blood concentration as corrected for weight. More frequent incidences of apnea, periodic breathing is well as obstruction following morphine has been reported in older patients relative to young adults. In neonates again with weight adjustment, morphine administration causes a greater degree of respiratory depression relative to the adult patient. This effect is probably due to a more ready penetration by morphine into the neonatal brain due to the underdeveloped blood-brain barrier. The sensitivity of morphine to the extent of blood brain barrier development is probably due to its relatively low intrinsic lipid-solubility which would normally be limiting. By contrast, neonatal patients are not more sensitive to other more lipid-soluble opioid such as fentanyl, sufentanil, or meperidine; the intrinsic lipid solubility then would be more important than the status of the blood-brain barrier.
1Effects of other drugs administered concurrently: opioid-induced respiratory depression would be in hand/prolonged if opioid administration is associated with administration of other CNS the presence which would include potent inhaled anesthetics, barbiturates, benzodiazepines, or ethanol. Bradypnea are apnea can be induced by these drug interactions which depress ventilatory responses to hypercarbia and/or hypoxia. Examples of agents which DO NOT enhance bradypnea or apnea in this setting include droperidol, scopolamine, and clonidine. This conclusion was reached by noting that these agents do not increase fentanyl or other opioid-induced respiratory depression.
1Pain: Respiratory depression may be influenced by pain and/or surgically-induced pain; however, some clinical research findings have not supported this assertion. (1) some postsurgical breathing patterns are not influenced by the level or type of pain relief. (2) sevoflurane-induced sedation that causes ventilatory response depression to hypoxemia is not reversed by acute pain.
1Opioids & Tolerance: Some degree of acute tolerance to respiratory depression secondary to opioid administration can be noted rapidly. However, a longer period, perhaps one half-year of opioid exposure may be required to detect significant tolerance to respiratory-depressant opioid action on hypoxic ventilatory responses. Complicating the consideration is the observation that cross-tolerance to the respiratory depression action of differing opioids may be unpredictable and incomplete.
Abnormal physiological renal function can translate to abnormal opioid activity duration. This result occurs despite the conclusion that opioid action is mainly ended by redistribution and hepatic metabolism. The general idea is that greater respiratory depression can follow from the accumulation of morphine/meperidine/metabolites in the renal insufficiency case.
Postoperative respiratory depression following fentanyl
(10 and 25 ![]() g/kg)
may be prolonged and enhanced by hypocapnic hyperventilation. The
opposite consequence is associated with interoperative hypercarbia.
These results may be explained by the following:
g/kg)
may be prolonged and enhanced by hypocapnic hyperventilation. The
opposite consequence is associated with interoperative hypercarbia.
These results may be explained by the following:
Increase brain opioid transfer to the brain would be expected with increased uncharged fentanyl (un-ionized) associated with the hypocarbic state
By contrast, increased removal may occur secondary to decrease cerebral blood flow with the hypocarbic state.
Another factor might be reduced hepatic clearance secondary to reduced cardiac output with attenuated hepatic blood flow.
1Bailey, PL, Egan, TD, Stanley, TH, "Intravenous Opioid Anesthetics", in Anesthesia 5th edition, Miller, R.D., editor, Churchill Livingstone, Philadelphia, 2000, 273-377 (references secondarily sourced from this primary reference are noted below in the indented references)
1aLowenstein, E, Hallowell P, Levine FH et al.: Cardiovascular response to large doses of intravenous morphine in man.N. Engl J Med 281:13 89, 1969
1b Stanley, TH, Webster LR; Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth Analg 51:901, 1972.
1c Arens, JR, Benbow, BP, Ochsner JL et al: Morphine anesthesia for the aorto-coronary bypass procedures. Anesth Analg 57: 411, 1978
1d Stanley, TH, Gray NJ, Staford W. et al: The effects of high-dose morphine on fluid and blood requirements in open-heart operations. Anesthesiology 38:536, 1973.
1e Stoelting, RK, Gibbs PS, Creasser CW et al: Hemodynamic in ventilatory response to fentanyl, fentanyl-droperidol, and nitrous oxide in patients with acquired valvular heart disease. Anesthesiology 42:319, 1975.
1f Bowdle, TA, Ward, RJ: Induction of anesthesia with small doses of sufentanil or fentanyl: Dose versus EEG response, speed of onset and thiopental requirement. Anesthesiology 70:26, 1989
1gHecker BR, Lake CL, DiFazio CA et al: The decrease of the minimum alveolar anesthetic concentration produced by sufentanil in rats. Anesth Anal 62:987, 1983.
1hGoldstein,A: Opiate receptors. Life Sci. 14:615, 1974.
1iSnyder SH: Opiate receptors in the brain. N Engl. J Med to 96:266, 1977.
1jMayer, DJ, Wolfle, TL, Akil H et al: Analgesia from electrical stimulation in the brainstem of the rat. Science 174:13 51, 1971.
1kFields, HL: Brainstem mechanisms of pain modulation: Anatomy and physiology. In Herz, A (ed): Opioids II: Handbook of Experimental Pharmacology. Berlin, Springer-Verlag, 1993, p.3.
1lKissin I, Vinik HR, Castillo R et al:Alfentanil potentiates midazolam-induced unconsciousness in subanalgesic doses. Anesth Analg 71:65, 1990
1mMcEwan AI, Smith C, Dyar O et al: Isoflurane minimum alveolar concentration reduction by fentanyl. Anesthesiology 78:864, 1993.
1nBailey, PL, Wilbrink J, Zwanikken P et al: in Anesthetic induction with fentanyl. Anesth. Analg 64:45, 1985.
1oHeier, T, Steen, PA: Assessment of anesthesia depth.Acta Anaesthesiol Scand 40:10 87, 1996.
1pSebel PS Lang E Rampil IJ White PF Cork R Jopling M Smith NT Glass PSA Manberg A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect Anesth. Analg. 84(4) 1997 891-899.
1qJones, JG: Use of evoked responses in the EEG to measure depth of anesthesia. In Lunn J, Rosen M (eds): Consciousness, Awareness and Pain and General Anesthesia. Boston, Butterworth, 1987, p 99.
1rEgan TD, Minto CF, Hermann DJ et al: Remifentanil versus alfentanil: Comparative pharmacokinetics and pharmacodynamics. Anesthesiology 84: 821, 1996.
1sKalkman CJ, Rheineck AR, Bovill JG: of high-does opioid anesthesia on posterior tibial nerve somatosensory cortical evoked potentials: Effects of fentanyl, sufentanil, and alfentanil J Cardiothorrac Anesth 2:758, 1998.
1tAdler, LJ, Fyuulai F, Diehl D, Mintun, M, Winter, PM, Firestone, L Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 1997; 84:120-126.
2Coda, BA, "Opioids" in Clinical Anesthesia, 4th edition, Barash, PG, Cullen, BF, Stoelting, RK, editors, Lipincott Williams & Wilkins, Philadelphia, 2001, 345-375
2a Thorpe, DH: Opiate structures and activity: a guide to underlying opioid actions. Anesth Analg 63:143, 1984.
3Gottschalk, A and Smith DS, New Concepts in Acute Pain Therapy: Preemptive Analgesia, American Family Physician, May, 2001 http://www.aafp.org/afp/20010515/1979.html
First figure redrawn with permission by Gottschalk and Smith from: Kehlet H, Dahl JB. The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesth Analg 1993;77:1049.
Second figure redrawn with permission by Gottschalk and Smith from: Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993;77:368.
4Dickenson, AH Spinal cord pharmacology of pain. Br. J. Anaesth. 75: 193, 1995. (references secondarily sourced from this primary reference are noted below in the indented references)
4aBattaglia, G, Rustioni A. Coexistence of glutamate and substance P. and dorsal root ganglion cells of the rat and monkey. Journal of Comparative Neurology 1988; 277:302-312.
4bHaley, JE, Sullivan, AF, Dickenson, AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rent. Brain Research 1990; 518:218-222.
4cSchiable, HG, Grubb BD, Neugebauer, V, Oppmann M. The effects of NMDA antagonists on neuronal activity in cat spinal cord evoked by acute inflammation in the knee joint. European Journal of Neuroscience, 1991; 3:981-991.
4dWoolf CJ, Thompson, SWN. The induction and maintenance of central sensitization is dependent on N--methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury hypersensitivity states. Pain 1991; 44:293-299.
4eMao, J, Price DD, Hayes, RL, Lu, J, Mayer DJ, Frank H. Intrathecal treatment with dextrophan or ketamine publicly reduces pain-related behaviors in a rat model of peripheral mononeuropathy. Brain Research 1993; 605:164-168.
4fPrice, DD, Mao, J, Mayer DJ. Central neural mechanisms of normal in abnormal pain states. In: Fields, HL, Lebeskind, JC, eds. Progress in Pain Research and Management. Seattle:IASP Press, 1994; 61-84.
4gRogawaki MA. Therapeutic potential of excitatory amino acid antagonists; channel blocks and 2,3 benzodiazepines. Trends in Pharmacological Sciences 1993; 14:325-331
4hEide PK, Jorum E, Stubhaug, A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartate receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain 1994; 58:347-354.
4iPrice DD, Mao J, Frenk H, Mayer, DF. Central neural mechanisms of normal in abnormal pain states. In:Fields HL, Lebeskind JC, eds. Progress in Pain Research and Management. Seattle:IASP Press, 1994; 61-84.
4jBesse, D, Lomabard, MC, Zajac, JM, Roques, BP, Besson, JM Pre- and and postsynaptic distribution of mu, delta, and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord, Brain Research 521 (1-2), June 1990 pp 15-22.
4kKangrga I and Randic M Outflow of endogenous aspartate in glutamate from the rat spinal dorsal horn in vitro by activation of low-and high-threshold primary afferent fibers. Modulation by mu-opioids Brain research 553, 2, July 1991,pp. 347-352.
5Substantia Gelatinosa Image: Courtesy of
The Digital Slice of Life, a cooperative project with the
Slice
of Life office, KUED
Media Solutions, and the Knowledge
Weavers Project.
Ross, AF, Gomez, MK, Tinker, JH "Anesthesia for Adult
Cardiac Procedures in Principles and Practice of Anesthesiology, 2nd
edition, Longnecker, DE, Tinker, JH, Morgan, GE, eds, Mosby, St. Louis,
1998, pp. 1659-1698.
6Two-dimensional & Three Dimensional Images courtesy of CORD Center for Opioid Research and Design http://www.opioid.umn.edu/
7Jaffe, CC, Stewart, WB, Lynch, PJ, Hines, S., Cranial Nerves, Yale University School of Medicine, Center for Advanced Instructional media, (c) 1998 http://info.med.yale.edu/caim/cnerves/contents.html
8Kauffman, AM & Patel M, Center for Cranial Nerve Disorders http://www.umanitoba.ca/cranial_nerves/trigeminal_neuralgia/manuscript/index.html
9Chakrabarti, S, Oppermann, M Gintzler AR Chronic morphine induces the concomitant phosphorylation and altered Association of multiple signaling proteins: A novel mechanism for modulating cell signaling PNAS 98 (7) 4009, 2001
10Brookoff, D Chronic Pain: 1. A New Disease? Hospital Practice July, 2000 http://www.hosppract.com/issues/2000/07/brook.htm
11Protein Structure Study Guide http://www.princeton.edu/~actin/chm543_info.html#Study%20guide%201
12MRC Center for Synaptic Plasticity, University of Bristol, used with permission for non-commercial applications
13Breeze AL, Harvey, TS, Bazzo, R, Campbell ID Solution structure of Human Calcitonin Gene-Related Peptide by H NMR and Distance Geometry with Restrained Molecular Dynamics Biochemistry 30:575-582 (1991)
14Heish, JC, Carr, DB "Choosing a Therapeutic Approach: Opioids" in The Massachusetts General Hospital Handbook of Pain Management (Borsook D, LeBel, AA, McPeek, B, eds) Little, Brown and Company, Boston, 1996, pp 47-75.
15Anwari, J. S. & Iqbal, S. (2003)Antihistamines and potentiation of opioid induced sedation and respiratory depression. Anaesthesia 58 (5), 494-495
3aGustafsson LL, Schildt B, Jacobsen KJ. Adverse effects of extradural and intrathecal opiates: Reports of a nationwide survey in Sweden. British Journal of Anaesthesia 1982; 54:479-486.
3bBrockways MS, Nobel DW, Sharwood-smith GH, McClure JH. Are found respiratory depression after extradural fentanyl. British Journal of Anaesthesia 1990; 64:243-245.
3cHolmstrom, B, Rawal N, Axelsson K, Nydahl P. Risk of catheter migration during combined spinal epidural block. Percutaneous epiduroscopy study. Anesthesia and Analgesia 1995; 80:747-753.
16Yli-Hankala,A Will enough isoflurane during surgery replace morphine after surgery? Acta Anaesthesiologica Scandinavica Volume 47 Issue 7 Page 785 - August 2003)
17Gurman GM, Weksler N, Steiner O, Popescu M, Avinoah E, Porath A. The influence of cortical electrical activity level during general anaesthesia on the severity of immediate postoperative pain in the morbidly obese. Acta Anaesthesiol Scand 2003; 47:804-8
17Soliman E Legatt, AD Somatosensory Evoked Potentials: General Principles eMedicine, http://www.emedicine.com/neuro/topic640.htm, 8/2001.
18Black,
S, Sloan T., SOMATOSENSORY (SSEP) AND MOTOR EVOKED POTENTIALS (MEP) THE
SOCIETY FOR NEUROANESTHESIA AND CRITICAL CARE - 1998 ANNUAL MEETING
http://analgesic.anest.ufl.edu/anest2/mahla/snacc/eps/