|
|
|
Medical Pharmacology Chapter 33-34: Anticancer Drugs
Miscellaneous Anticancer Drugs:
References
|
|
Numerous metabolic differences have been described when comparing malignant to normal cells.12
Some differences are related to metabolic dependencies on certain enzyme systems.
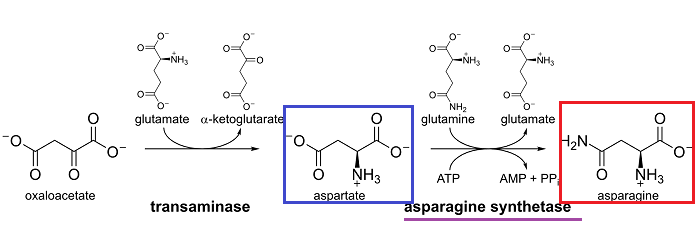
For example, in lymphoid cells (ALL) a dependence on an exogenous amino acid, asparagine resulted in development of L-asparaginase (L-ASPA) for use in treating childhood acute lymphoblastic leukemia (ALL)
L-asparagine is formed by transamination of L-aspartic acid.12
 |
|
Although the enzyme L-asparagine synthetase (L-ASPS) is typically found in tissues, in the case of human lymphocytic malignancies the ability to synthesize asparagine is not present.
Accordingly, L-asparagine would have to be obtained from other sources, such as blood.
![]() At all
events, the enzyme asparaginase (L-ASPA, L-asparagine aminohydrolase)
hydrolyzes asparagine to aspartic acid (and ammonia) and this enzyme has
become an important agent in managing childhood and adult acute
lymphoblastic leukemia (ALL).12
At all
events, the enzyme asparaginase (L-ASPA, L-asparagine aminohydrolase)
hydrolyzes asparagine to aspartic acid (and ammonia) and this enzyme has
become an important agent in managing childhood and adult acute
lymphoblastic leukemia (ALL).12
|
|
|
Normal tissues usually synthesize L-asparagine.1
However, lymphocytic leukemia exhibits reduced levels of the synthetic enzyme, asparagine synthase, to meet cellular requirements.
Therefore these malignant cells must obtain sufficient asparagine from blood or other sources.
![]() Asparaginase
reduces available extracellular L-asparagine by catalyzing
hydrolysis of asparagine to aspartic acid and ammonia.
Asparaginase
reduces available extracellular L-asparagine by catalyzing
hydrolysis of asparagine to aspartic acid and ammonia.
As a result, malignant cells are unable to obtain adequate asparagine levels and cell death ensues.1
Also, asparaginase may also exhibit antineoplastic activity by depleting essential glutamines stores (a glutaminase effect) that results in DNA biosynthesis inhibition.10
Glutaminase activity may be associated with side effects including:
Pancreatitis
Homeostatic abnormalities
Thrombotic complications
Neurologic complications.10
|
|
|
|
|
|
Absorption, Distribution, Biotransformation, Excretion:
The route of administration of asparaginase is intravenous (IV) or intramuscular (IM).1
E. coli-derived asparaginase exhibits a plasma clearance rate of about 0.035 ml/min/kg and a volume of distribution (Vd) approximately equivalent to human plasma volume.1
Blood plasma represents about 55% of total blood volume.17
In males, blood plasma volume approximates 39 mL/kg of body weight.
In females, plasma volume is about 40mL/kg.17
The plasma half-life of asparaginase (t1/2) is about 24-30 hours (following IV administration).1,12
The plasma elimination half-life of asparaginase (t1/2) increases to about 35-50 hours following intramuscular administration.9
Time to peak plasma levels for asparaginase derived from E. coli and after intramuscular administration is about 20 hours.
A substantially longer plasma half-life (t1/2) is associated with a pegylated form of asparaginase, Pegaspargase which involves conjugation of asparaginase to 5000 Dalton (Da) units of monomethoxy polyethylene glycol.
With pegaspargase, plasma half-life is extended to about 6-7 days with intramuscular administration every two weeks.
Pegaspargase is especially useful in patients who exhibit hypersensitivity to asparaginase because pegaspargase is not immunogenic in most patients (70%).
For patients who exhibit hypersensitivity reactions to the E. coli-based L asparaginase preparations an alternative is the Erwinia (from Erwinia chrysanthemi) enzyme.
 |
|
![]() Pegaspargase
administration is associated with rapid and near-complete plasma in tumor
cell asparagine depletion for about three weeks in most patients.1
Pegaspargase
administration is associated with rapid and near-complete plasma in tumor
cell asparagine depletion for about three weeks in most patients.1
Asparaginase is frequently used in combination with other agents for treatment of acute lymphoblastic (lymphocytic) leukemia and high-grade lymphomas.
![]() Resistance to
asparagine appears to be associated with induction of the enzyme asparagine
synthetase in the cancer cell.1
Resistance to
asparagine appears to be associated with induction of the enzyme asparagine
synthetase in the cancer cell.1
Adults diagnosed with acute lymphoblastic leukemia undergo induction therapy using a combination of:
Vincristine
Prednisone
an Anthracycline
Asparaginase
Cyclophosphamide (on occasion).20
![]() In adults,
consolidation treatment following remission is intensive with some protocols
using
In adults,
consolidation treatment following remission is intensive with some protocols
using
Methotrexate (high-dose)
Cytarabine
Asparaginase.20
![]() In the pediatric
patient diagnosed with acute lymphoblastic leukemia, remission induction
protocols typically involve:21
In the pediatric
patient diagnosed with acute lymphoblastic leukemia, remission induction
protocols typically involve:21
Glucocorticoid (prednisone, dexamethosone, prednisolone)
Vincristine
Asparaginase +/- Daunorubicin.
Pediatric Remission induction protocols typically include asparaginase.
Different forms of asparaginase exhibit differing pharmacocokinetics as well as differences in efficacy and toxicity.
For example, compared with E. coli asparaginase, Erwinia asparaginase may exhibit poorer antileukemic responses.21
![]() Two major
toxicity groups are associated with L asparaginase administration.1,12
Two major
toxicity groups are associated with L asparaginase administration.1,12
(1) Toxicities secondary to asparagine depletion and reduced protein synthesis and
(2) Toxicities stemming from immunologic reactions to the foreign protein, asparaginase.
Hypersensitivity responses to asparaginase are both common and associated with asparaginase inactivation.
![]() These
reactions may be fatal.
These
reactions may be fatal.
![]() When
asparaginase is used in monotherapy, hypersensitivity reactions are more
likely.
When
asparaginase is used in monotherapy, hypersensitivity reactions are more
likely.
In this single-agent treatment setting, sensitization may be noted in up to 40% of patients receiving the drug.
In combination protocols involving asparaginase, the concurrent presence of corticosteroids, 6-mercaptopurine as well as other antileukemic drugs is likely responsible four reduced hypersensitivity incidents (<20%).12
In those patients susceptible to asparaginase hypersensitivity responses, an effective and safe alternative is offered by pegaspargase.
![]() Hypersensitivity
reactions may be "silent" in that antibody-mediated asparaginase
inactivation may happen in patients not exhibiting clinically observable
hypersensitivity. In this case adverse clinical outcome may occur, notably
in ALL high-risk individuals.1
Hypersensitivity
reactions may be "silent" in that antibody-mediated asparaginase
inactivation may happen in patients not exhibiting clinically observable
hypersensitivity. In this case adverse clinical outcome may occur, notably
in ALL high-risk individuals.1
Hypersensitivity to asparaginase is associated with various clinical responses.12
About 2/3 of hypersensitivity reactions (reported) involve urticaria (itching).
Other reactions include dyspnea along with major anaphylactic responses ("hypotension, laryngospasm, and cardiac arrest").
![]() Notably, the
occurrence of hypersensitivity reactions represents an indication of
asparaginase inactivation which likely predisposes to an adverse clinical
result.
Notably, the
occurrence of hypersensitivity reactions represents an indication of
asparaginase inactivation which likely predisposes to an adverse clinical
result.
![]() Fatal
reactions to E. coli asparaginase are infrequent (<1%).
Fatal
reactions to E. coli asparaginase are infrequent (<1%).
However, an indication of hypersensitivity should be sufficient to change from E. coli asparaginase to Erwinia asparaginase.
Allergic reactions to Erwinia asparaginase may also occur in patients not previously treated with the E. coli enzyme.12
Toxicities resulting from protein synthesis inhibition due to inadequate asparagine levels as a result of asparaginase activity include:1
Hyperglycemia secondary to insulin deficiency
Clotting abnormalities as a result of inadequate clotting factors
![]() The effect on
clotting may in the extreme present as intracranial hemorrhage soon after
asparaginase treatment initiation.
The effect on
clotting may in the extreme present as intracranial hemorrhage soon after
asparaginase treatment initiation.
Fortunately, this adverse effect is classified as "infrequent".
Hypertriglyceridemia as a result of aberrant lipoprotein production
Hypoalbuminemia.1