Nursing Pharmacology Chapter 1: General Principles: Overview and Introduction
Chiral refers to molecule with a center of three-dimensional asymmetry.
Definiton: Chirality- "handedness"; (Greek cheir - the hand); molecules which are not superimposable on (cannot be made to coincide with) their mirror image. achiral molecules can be superimposed on its mirror image.
![]() Many drugs, over 50% of all drugs actually, are chiral (existing as enantiomeric pairs)
Many drugs, over 50% of all drugs actually, are chiral (existing as enantiomeric pairs)
Enantiomers (molecules having opposite shapes) are pairs of molecules existing in forms that are mirror images of each other (right-& left-hand) but that cannot be superimposed
Other than lack of superimposition, enantiomers are chemically identical, but may be distinguished by the direction in which they rotate polarized light, either dextro (d or +) or levo (l or -).
Plane-Polarized Light Rotation: Plane-polarized light is light in which all wave vibrations have been filtered out except for those in one plane.
Passing light through a polarized lens allows only one wave direction to be transmitted.
When the plane-polarized light wave is then passed through a solution containing a single enantiomer of a compound the light is rotated by a certain amount either clockwise or counter clockwise.
"Optical Rotation is the rotation of plane-polarized light.
Optically active compounds are chiral compounds which possess the ability to rotate plane-polarized light.
A polarimeter is an instrument which polarizes light and then shows the angle of rotation of plane-polarized light by an optically active compound.
Enantiomers present in equal proportion (50:50) are referred to as racemates.
|
Ways looking at chirality
Chirality has to do with "right or left handedness"
If an item appears identical to its image in a mirror it is said to be "achiral" -- an example would be a water glass.
The basic issues a comparison between an object and its reflection -- if an object is different from its reflection it is said to be chiral; for instance, ones left-hand and right hand are chiral.
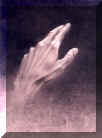 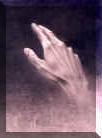 |
Note also that when you put your left-hand up to mirror, the image that appears is a right hand, thus illustrating that left and right hands are in fact mirror images of each other.
Note the structure of thalidomide:
|
|
Thalidomide is chiral, i.e. left and right-handed molecular forms are present.
One form produced sedation.
The other form was responsible for fetal abnormalities.
![]() Although
an important therapeutic use for thalidomide today
is in treatment of leprosy, in particular a
disease complication called erythema nodosum
leprosum, thalidomide derivatives appear effective in
treating certain cancers, such as multiple
myeloma.
Although
an important therapeutic use for thalidomide today
is in treatment of leprosy, in particular a
disease complication called erythema nodosum
leprosum, thalidomide derivatives appear effective in
treating certain cancers, such as multiple
myeloma.
Anti-cancer mechanisms include:
(1) Reduction in inflammatory proteins including tumor necrosis factor alpha (TNF-α),
(2) Immune system modulation and
(3) An anti-angiogenesis property which blocks formation of new blood vessels. Tumor growth is dependent on angiogenesis.
Usually one enantiomer will be more effective than its mirror structural image, suggesting a more complementary fit between the drug with its receptor binding region.
Examples:
S(+)- methacholine enantiomer > 250 times more potent than the R(-) enantiomer form
Ketamine (Ketalar): (+) enantiomer more potent, less toxic then the (-) enantiomer; however, the drug is used as the racemate (includes both enantiomeric forms)
Some enantiomeric differences are important in anesthesia-related medications:
Cardiotoxicity associated with bupivacaine is probably due to the d-bupivacaine (Marcaine) isomer, perhaps because d- bupivacaine (Marcaine) occupies the sodium channel longer than l- bupivacaine (Marcaine).
Ropivacaine (Naropin), related to bupivacaine (Marcaine), is synthesized as a single enantiomer, exhibiting decreased cardiotoxicity.
Cisatracurium (Nimbex), an atracurium (Tracrium) isomer, does not promote histamine release.
d- propranolol (Inderal) is the form than blocks beta-adrenergic receptors; however, both enantiomers exhibit "local-anesthetic" effects.
The antiarrhythmic properties of propranolol (Inderal) therefore, depending on concentration, may include both beta adrenergic blockade, at lower concentrations (d-propranolol (Inderal)), as well as a direct membrane effect which is not require stereoselectivity.
Dexmedetomidine (Precedex), which has been approved by the FDA for sedation of critically ill patients in the ICU setting, is a good example of a drug which, in the l-, form has no effect on halothane (Fluothane) MAC (Minimum Alveolar Concentration necessary to prevent patient response to painful stimulation in 50% of the population); however, the d- form dramatically reduces halothane (Fluothane) MAC.
Dexmedetomidine (Precedex), presently used (on-label) in the ICU setting, exhibits the ability to produce a state of patient "tranquility"in which patients appear asleep but are easily aroused and exhibit no significant respiratory depression.
Naturally occurring drugs are typically stereospecific because enzymes involved in their synthesis exhibit stereoselectivity:
Examples:
l- morphine
d- tubocurarine
3Mechanistic implications of enantiomer-selective biological effects:
Concerning the mechanisms of anesthetic action, an important aspect was derived from the observation that there was a significant correlation between anesthetic potency and lipid solubility (Meyer-Overton rule).
Generally, this rule had been applied to inhaled anesthetics rather than intravenous drugs. Lipid solubility is an important physical-chemical property of drugs that is important in getting the drug to the site of action (lipid bilayer-based membrane structure).
How anesthetics work however does not appear to be explained solely on the basis of lipid solubility.
A clue comes from the observation that some anesthetics exhibit some degree of enantiomer-selectivity.
Examples of such agents include barbiturates, etomidate (Amidate), and isoflurane (Forane). Enantiomer-selectivity suggests a protein (receptor) involvement since presumably proteins exhibit the degree of molecular specificity required to select preferentially one enantiomer from another in terms of biological efficacy.
A more general point, is that the way biological specificity works depends upon precise 3-D alignment of molecular groups and therefore is intrinsically, most of the time, stereoselective -- that is, preferring one stereoisomer over the other.
Such selectivity is reflected not only in one drug enantiomeric form being either more potent or less potent than another but also in the body prefers one amino acid isomer over another (typically the in l- form).
Recall that this specificity is embedded at the deepest levels of enzyme, transport proteins, and nucleic acid interactions with biomolecules.
3Examples of anesthetic interaction/receptor sites:
Transmitter-gated ion channels
GABA (gamma-aminobutyric acid) and glycine receptors
At anesthetic concentrations, all intravenous anesthetic drugs enhance GABAa (subtype a) receptor-mediated effects. Recall that GABA systems are inhibitory
Some anesthetic agents inhibits excitatory responses associated with nicotinic, cholinergic receptor activation, whereas others inhibits ionotropic glutamate receptor types (glutamate is an excitatory neurotransmitter)
Anesthesia action may be based on both activating inhibitory neurotransmitter-receptor CNS systems and inhibiting excitatory neurotransmitter-receptor CNS systems.
Katzung, B. G. "Basic Principles: Introduction" in Basic and Clinical Pharmacology, (Katzung, B. G., ed) Appleton-Lange, 1998, p.1-4
Stoelting, R.K., "Pharmacokinetics and Pharmacodynamics of Injected and Inhaled Drugs", in Pharmacology and Physiology in Anesthetic Practice, Lippincott-Raven Publishers, 1999, 1-17
3Dolin, S. J. "Drugs and pharmacology" in Total Intravenous Anesthesia, pp. 13-35 (Nicholas L. Padfield, ed), Butterworth Heinemann, Oxford, 2000