|
|
|
Nursing Pharmacology Chapter 33-34: Anticancer Drugs
Natural Products: Vinca Alkaloids
Vinblastine (Velban,VBL)
Vinblastine is an anticancer drug derived from Vinca rosea, the periwinkle plant.3
Vinca alkaloids manifests their anticancer effect by interfering with microtubule function by means of tubulin polymerization inhibition.
Since microtubules are essential for proper mitotic spindle function, vinca alkaloids administration results in mitotic arrest in metaphase.
![]() As
a consequence cell division is halted and cell death ensues.3
As
a consequence cell division is halted and cell death ensues.3
Absorption, Distribution, Biotransformation and Excretion:
Absorption:
Vinblastine sulfate is a vesicant (causes blistering) and as such is administered by the IV route of administration.
Special care should be used to prevent subcutaneous extravasation which may induce significant irritation and ulceration.
|
|
|
![]() Because these agents are vesicants there is a risk of
phlebitis even with proper IV administration.11
Because these agents are vesicants there is a risk of
phlebitis even with proper IV administration.11
As a result, the vein should be flushed following vinblastine administration.
This precaution would apply to the other vinca alkaloids as well.
Should extravasation occur or if extravasation is suspected several courses of action appear appropriate:
1. Treatment discontinuation.
2. Aspiration of residual vinblastine (or whichever vinca alkaloid/derivative which might have been used) remaining in the tissue.
3. Application of heat.
Reduced discomfort and reduced risk of latent cellulitis may benefit from hyaluronidase injection into surrounding tissue.
Early surgical debridement following extravasation may be appropriate.11
| Alkylating agents | Antitumor antibiotics | Anthracyclines | Taxane | Vinca alkaloids | Non-classical alkylating agent |
|
|
|
|
|
|
Vinblastine and the other vinca alkaloids are metabolized by the liver microsomal drug metabolizing system also known as the hepatic cytochrome P450 metabolizing system.8,11
Vinblastine is rapidly taken up by tissue and exhibits significant binding to both plasma proteins and formed blood elements.
Disappearance of vinblastine from plasma is characterized by a triexponential model.8
In this model the initial rapid distribution phase exhibits a t1/2 (half-time) of less than 5 minutes.
The final or terminal phase extends for much longer period of time with a t1/2 of about 24 h
Vinblastine and related agents utilize the liver cytochrome P450 system for metabolism, with metabolites excreted in the bile and ultimately feces (hepatobiliary system).11
A limited fraction (<15%) may be recovered in the urine, unchanged.
Because of the dependence on the liver for metabolism (detoxifying), patients with reduced liver function may require a reduction in vinca alkaloid dose.
![]() For
example, using bilirubin levels as a marker of hepatic
function, a bilirubin level >3 mg/dL amy require a
50%-75% dosage reduction of vinblastine or other vinca
alkaloids.
For
example, using bilirubin levels as a marker of hepatic
function, a bilirubin level >3 mg/dL amy require a
50%-75% dosage reduction of vinblastine or other vinca
alkaloids.
In general vinca alkaloids promote microtubule depolymerization with destruction of mitotic spindles.
Vinblastine administration at low but clinically effective concentrations does not appear to depolymerize spindle microtubules but does effectively block mitosis.
Vinca alkaloids bind to a particular region of the β-subunit of tubulin dimers, the vinca-binding domain.
![]() Alterations
of microtubule dynamics block mitotic progression.
Alterations
of microtubule dynamics block mitotic progression.
Abnormalities of normal mitotic spindle assembly delay progression of the cell cycle.
Chromosomes being immobilized at mitotic spindle poles are not able to transition from metaphase into anaphase.
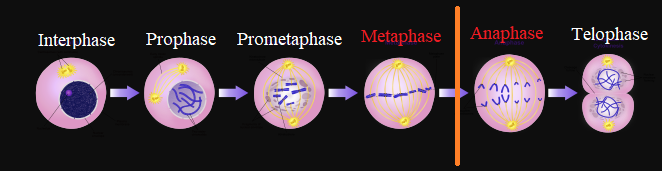 |
|
This condition triggers "programmed cell death", apoptosis.
These mechanisms apply generally to vincristine, vinblastine, as well as to VRL and VFL.
Vinblastine has several clinical antineoplastic clinical indications including:11
Hodgkin's and non-Hodgkin's lymphoma
Kaposi's sarcoma
Breast cancer
Bladder cancer
Prostate cancer
Testicular cancer
Renal cell cancer11
Testicular cancer:1
Vinblastine may be used in combination with two other drugs, bleomycin and cisplatin, a protocol providing curative treatment in patients exhibiting metastatic testicular cancer.1
Recently, etoposide or ifosphamide have tended to supplant vinblastine in this setting.1
Hodgkin's lymphoma:1
With respect to Hodgkin's lymphoma, vinblastine is a part of the "ABVD" treatment protocol.
This protocol consists of doxorubicin + bleomycin + vinblastine + dacarbazine administered in a number of treatment cycles depending on clinical assessment of disease.1
Additional uses for vinblastine include treatment of neuroblastoma, choriocarcinoma, and Langerhans cell histiocytosis.1
Major toxicities associated with vinca alkaloids administration differ depending on the particular drug employed.8
For example, with vincristine neurotoxicity describes the major toxicity.8
![]() By
contrast, vinblastine (VSBL), vindesine (VDS) bifluorinated
vinflunine (VFL) and vinorelbine (VRL) exhibit myelosuppression as
the primary toxicity.
By
contrast, vinblastine (VSBL), vindesine (VDS) bifluorinated
vinflunine (VFL) and vinorelbine (VRL) exhibit myelosuppression as
the primary toxicity.
With respect to vinblastine (VBL) [as well as for VDS, VRL, and VFL] neutropenia represents the primary dose-limiting toxicity.8
Less common and less severe hematologic toxicities are thrombocytopenia and anemia.
Neutropenia is typically observed clinically in the 7-11 day timeframe after treatment.
Recovery from neutropenia is noted within 2 to 3 weeks.
Vinblastine and the other agents exhibit some gastrointestinal autonomic dysfunction.8
This effect is most commonly noted with vincristine.
Manifestation of gastrointestinal effects include bloating, ileus, constipation and abdominal pain.
Paralytic ileus most commonly noted in pediatric patients as well as intestinal necrosis and perforation have been described.
Impaction of stool in the upper colon due to poor intestinal transit may also be noted.
![]() For
patients receiving vincristine measures to prevent constipation is
recommended.
For
patients receiving vincristine measures to prevent constipation is
recommended.
Severe ileus is more likely in patients receiving high vincristine dosage or in patients with liver dysfunction.
Mucositis, pharyngitis and stomatitis are more likely to be associated with vinblastine compared to VRL or VDS and is unlikely to be noted with vincristine.8