|
|
|
Nursing Pharmacology Chapter 33-34: Anticancer Drugs
Natural Products: Vinca Alkaloids
Vincristine (Oncovin, Vincasar, VCR)
Vincristine is an anticancer drug derived from Vinca rosea, the periwinkle plant.3
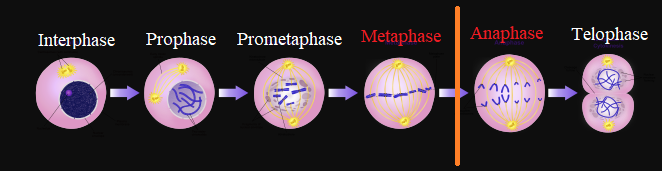
Vinca alkaloids manifests their anticancer effect by interfering with microtubule function by means of tubulin polymerization inhibition.
Since microtubules are essential for proper mitotic spindle function, vinca alkaloids administration results in mitotic arrest in metaphase.
![]() As
a consequence cell division is halted and cell death ensues.3
As
a consequence cell division is halted and cell death ensues.3
Although both vinblastine and vincristine are vinca alkaloids, vincristine shows a notably different clinical and safety profile.3
This difference appears most likely attributed to the higher affinity that vincristine exhibits for axonal microtubules.
Absorption, Distribution, Biotransformation, Excretion:
Absorption:
Vincristine is a vesicant (causes blistering) and as such is administered by the IV route of administration.
Special care should be used to prevent subcutaneous extravasation which may induce significant irritation and ulceration.
|
|
|
![]() Because these agents are vesicants there is a risk of
phlebitis even with proper IV administration.10
Because these agents are vesicants there is a risk of
phlebitis even with proper IV administration.10
As a result, the vein should be flushed following vincristine administration.
This precaution would apply to the other vinca alkaloids as well.
Should extravasation occur or if extravasation is suspected several courses of action appear appropriate:
1. Treatment discontinuation.
2. Aspiration of residual vincristine (or whichever vinca alkaloid/derivative which might have been used) remaining in the tissue.
3. Application of heat.
Reduced discomfort and reduced risk of latent cellulitis may benefit from hyaluronidase injection into surrounding tissue.
Early surgical debridement following extravasation may be appropriate.10
| Alkylating agents | Antitumor antibiotics | Anthracyclines | Taxane | Vinca alkaloids | Non-classical alkylating agent |
|
|
|
|
|
|
Distribution:8
Usual clinical doses of vincristine (VCR) are administered by the intravenous (IV) route of administration.
Vincristine exhibits substantial binding affinity for plasma proteins, exhibiting binding in the range of 50% to 75%.8
Vincristine also binds to blood elements such as platelets, a blood element characterized by high tubulin concentrations.
Vincristine does not readily cross the blood-brain barrier with cerebrospinal fluid exhibiting a 20 to 30-fold lower vincristine levels compared to that in plasma.
Following IV administration, vincristine distributes to bodily tissues rapidly and exhibits a triphasic elimination profile.
The terminal half-life is about 85 hours, consistent with a large volume of distribution and slow transfer from tissue compartments.8
Metabolism and Excretion:8
Vincristine is both metabolized and excreted by the hepatobiliary system.
About 10% of vincristine is excreted in the urine mainly as metabolites.
About 70%-80% is excreted in the feces (about 50% as metabolites).
![]() The hepatic
microsomal drug metabolizing system is responsible for vincristine
metabolism, principally dependent on the cytochrome P450 CYP3A isoform
family.
The hepatic
microsomal drug metabolizing system is responsible for vincristine
metabolism, principally dependent on the cytochrome P450 CYP3A isoform
family.
The primary metabolite is a secondary amine (M1) and is catalytically dependent on the CYP3A5 isoform.
![]() Genetic
differences in the expression of CYP3A5 could contribute to variability in
clinical response to vincristine.
Genetic
differences in the expression of CYP3A5 could contribute to variability in
clinical response to vincristine.
![]() The
dependency on the liver for vincristine detoxification is the basis for
cautious use of vinca alkaloids generally in patients with hepatic
dysfunction.
The
dependency on the liver for vincristine detoxification is the basis for
cautious use of vinca alkaloids generally in patients with hepatic
dysfunction.
![]() Antiproliferative effects of vincristine and other vinca alkaloids appear
dependent on both drug effects on microtubule stabilization and on
microtubule depolymerization.8
Antiproliferative effects of vincristine and other vinca alkaloids appear
dependent on both drug effects on microtubule stabilization and on
microtubule depolymerization.8
Both of these effects result in mitotic arrest.
These effects are noted at drug concentrations that induce no appreciable microtubule depolymerization or mitotic spindle disorganization.
At higher concentrations, microtubule organization and chromosomal organization in arrested mitotic spindles degrades in a way shared by all vinca derivatives.8
Antiproliferative effects of vinca alkaloids may be due to loss of dynamic stability as well as from literal microtubular depolymerization.
Despite the commonality of vinca agents with respect to mechanisms of action, differences may be due to varying affinities for the tubulin binding site.
For example, vincristine exhibits higher affinity compared to vinblastine.
The underlying mechanisms accounting for differences among various vinca alkaloids with respect to anti-proliferative action as well as toxicities await further investigation.8
Vinca alkaloids bind to a particular region of the β-subunit of tubulin dimers, the vinca-binding domain.
![]() Alterations
of microtubule dynamics block mitotic progression.
Alterations
of microtubule dynamics block mitotic progression.
Abnormalities of normal mitotic spindle assembly delay progression of the cell cycle.
 |
|
Vincristine has several clinical antineoplastic clinical indications including:1
Non-Hodgkin's lymphoma
Leukemias
Wilms tumor
Neuroblastoma
Rhabdomyosarcoma
Large Cell non-Hodgkin's lymphoma:1
With respect to Hodgkin's lymphoma, vincristineis a part of the "CHOP" treatment protocol.
This protocol consists of cyclophosphamide + doxorubicine + vincristine .1
Vincristine is part of other widely used chemotherapeutic protocols that exhibit significant activity including EPOCH, a combination of etoposide, doxorubicin, and vincristine given on certain days with oral prednisone on other days along with courses of IV cyclophosphamide.8
Major toxicities associated with vinca alkaloids administration differ depending on the particular drug employed.8
For example, with vincristine neurotoxicity describes the major toxicity.8
The neurotoxicity associated with vincristine encompasses both and autonomic polyneuropathy as well as a peripheral mixed sensorimotor neuropathy.10
These neuropathologies are associated with a variety of clinical symptoms including: constipation, paralytic ileus, urinary retention, abdominal cramping, orthostatic hypotension and hypertension.
Paralytic ileus most commonly noted in pediatric patients as well as intestinal necrosis and perforation have been described. 8
Impaction of stool in the upper colon due to poor intestinal transit may also be noted.
![]() For
patients receiving vincristine measures to prevent constipation is
recommended. 8
For
patients receiving vincristine measures to prevent constipation is
recommended. 8
The underlying mechanism for vincristine neuropathologies appears related to drug effects on axonal microtubules.
![]() Some
neuropathologies evolve in the direction of increasing
seriousness.10
Some
neuropathologies evolve in the direction of increasing
seriousness.10
For example an early presentation may be symmetric sensory impairment with parasthesias which in turn lead to neuritic pain and subsequent loss of deep tendon reflexes, during vincristine treatment.
Continued drug administration may then lead to foot drop, wrist drop, motor dysfunction, ataxia and ultimately paralysis.10
|
|
![]() By
contrast, vinblastine (VSBL), vindesine (VDS) bifluorinated
vinflunine (VFL) and vinorelbine (VRL) exhibit myelosuppression as
the primary toxicity.
By
contrast, vinblastine (VSBL), vindesine (VDS) bifluorinated
vinflunine (VFL) and vinorelbine (VRL) exhibit myelosuppression as
the primary toxicity.
With respect to vinblastine (VBL) [as well as for VDS, VRL, and VFL] neutropenia represents the primary dose-limiting toxicity.8
Less common and less severe hematologic toxicities are thrombocytopenia and anemia.
Neutropenia is typically observed clinically in the 7-11 day timeframe after treatment.
Recovery from neutropenia is noted within 2 to 3 weeks.
Vinblastine and the other agents exhibit some gastrointestinal autonomic dysfunction.8
This effect is most commonly noted with vincristine.
Manifestation of gastrointestinal effects include bloating, ileus, constipation and abdominal pain.
Paralytic ileus most commonly noted in pediatric patients as well as intestinal necrosis and perforation have been described.
Impaction of stool in the upper colon due to poor intestinal transit may also be noted.
![]() For
patients receiving vincristine measures to prevent constipation is
recommended.
For
patients receiving vincristine measures to prevent constipation is
recommended.
Severe ileus is more likely in patients receiving high vincristine dosage or in patients with liver dysfunction.
Mucositis, pharyngitis and stomatitis are more likely to be associated with vinblastine compared to VRL or VDS and is unlikely to be noted with vincristine.8