|
|
|
Anesthesia Pharmacology Chapter 4: Physics and Anesthesiology
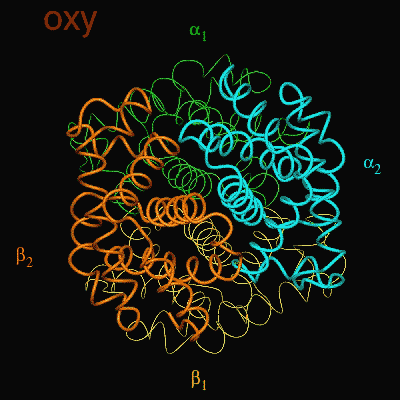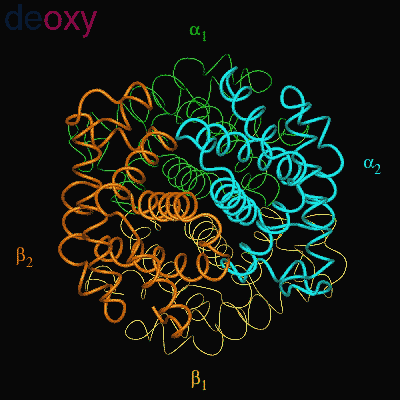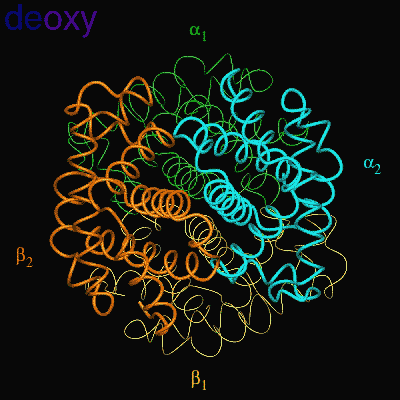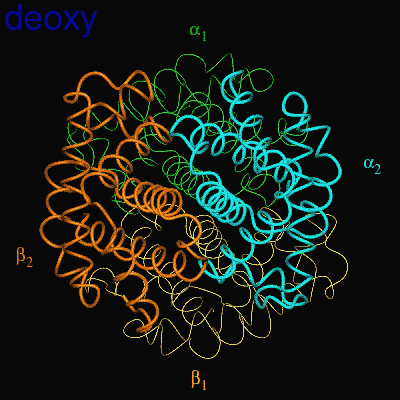 |
 |
 |
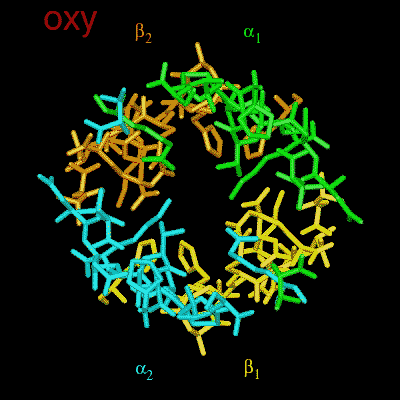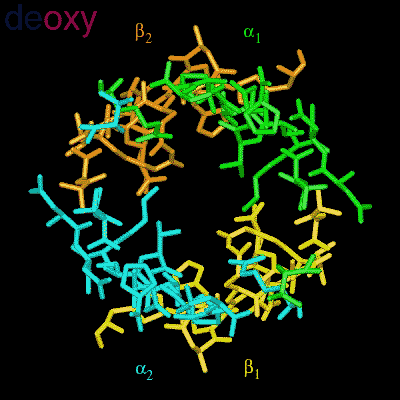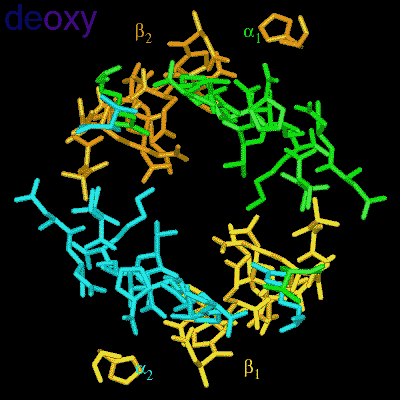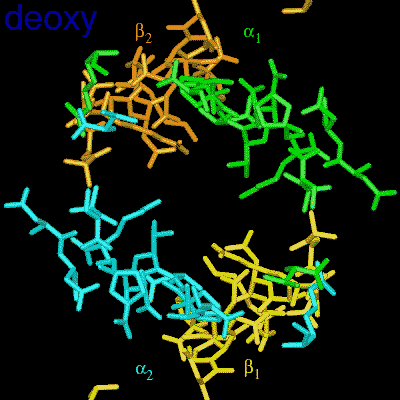 |
Residues, within 8 Å of the two-fold axis of Hb, are represented. These residues define a central cavity in hemoglobin and this cavity enlarges dramatically upon deoxygenation. Note the motion of the C-terminal Histidines of ß1and ß2 , towards the upper and lower edges of the figure, respectively. The C-terminal Arginines of the α subunits also move apart significantly, towards the upper left and lower right corners, in front of the subunits in the figure.
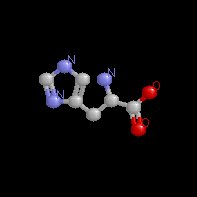
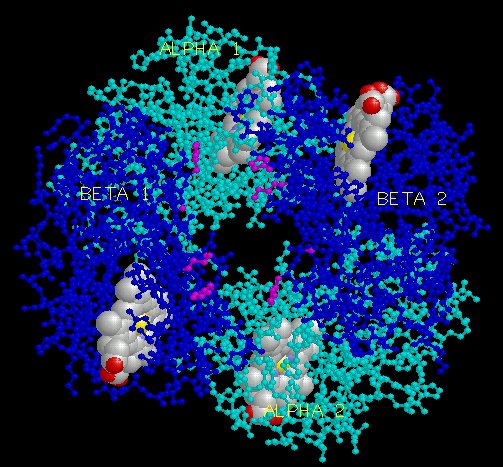
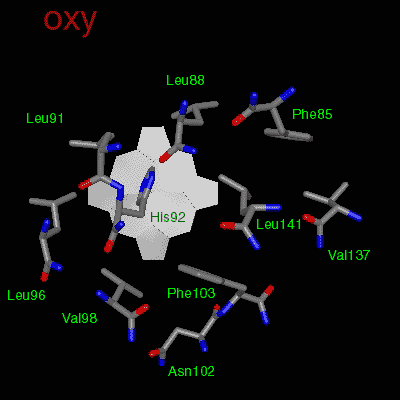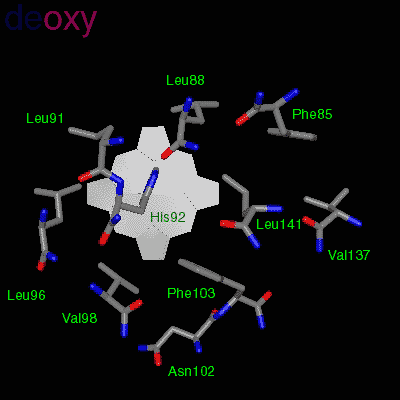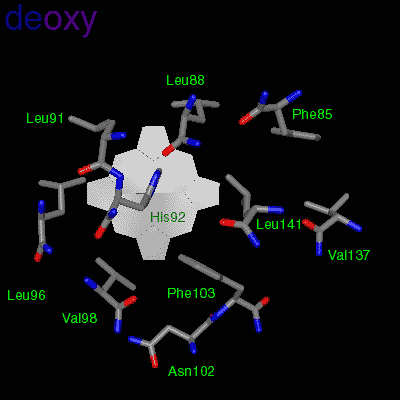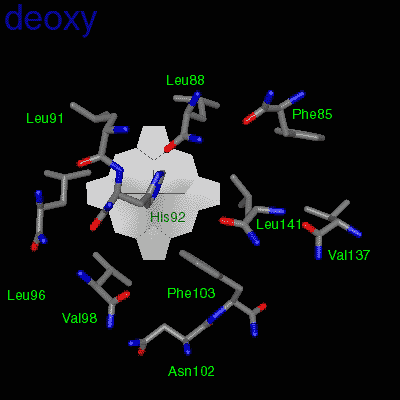
The effector (2,3)-DPG binds to positively-charged subunit residues in the central cavity domain. While (2,3)-DPG is too large to fit into the narrow cavity of oxy-Hb, it binds readily in the larger cavity of the deoxy conformation. Thus, (2,3)-DPG binding lowers the oxygen affinity of Hb by shifting the oxy-deoxy structural distribution of the protein in a manner that favors the deoxy state. The physiological consequence of 2,3 DPG modulation has been discussed above. Animation and description courtesy of Dr. John Lukin, Dept. of Biological Sciences, Carnegie Mellon University, used with permission.
 |
 |
|
|
![]()
 |
54Dr. John Lukin, Dept. of Biological Sciences, Carnegie Mellon University