|
|
|
Anesthesia Pharmacology Chapter 4: Physics and Anesthesiology
More about Protein Secondary Structure
As noted before, the sequence of amino acids which comprise the primary structure the protein do not remain as elongated strings, but rather fold into more complex structures.
The first step in this process is the conversion of the protein from a primary structure to a secondary structure.
The principal secondary structures, in terms of classification, include the alpha helix and the beta (pleated) sheet.
Factors that induce these more complex three-dimensional structures include interactions between individual amino acids side chains manifest in terms of hydrogen bonding and hydrophobic interactions.
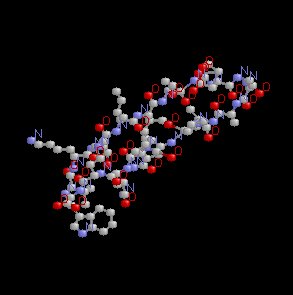
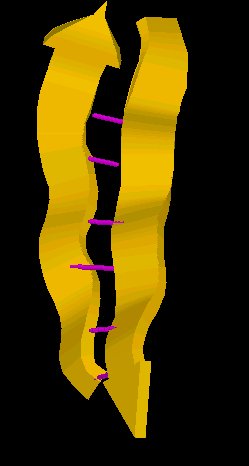
As shown below, the alpha helix, which appears to wind up as the structure progresses, exhibits flexibility and stability and might be expected to be an important component of a protein which must exhibit flexibility.
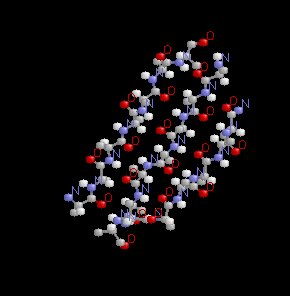
By contrast to the alpha helical form, the beta sheet is based on the structure in which to amino acid chains adjust, one relative to the other such that H bonds form between juxtaposed amino acids. Examples of this more beta rigid structure are given below.
 |
 |
 |
In any single given protein there may be regions of the protein that are alpha helical in nature and other regions that are in the beta form.
The alpha and beta structures are the principal repeating elements in a protein and the interrelationships between such repeating elements define a higher order structure called the tertiary structure.
Although the
alpha helical and data confirmations are the major repeating elements, an
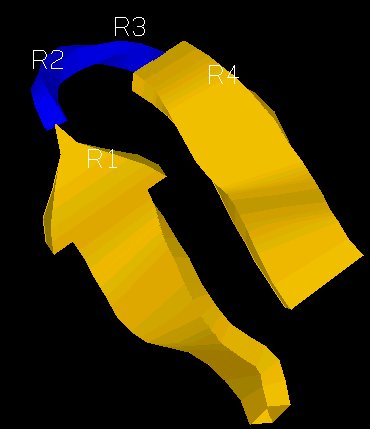
additional one called a beta turn or bend is important because it allows for
very abrupt changes in direction of the polypeptide chain. The beta bend
with its abrupt turn typically links adjacent antiparallel beta-sheets. An example is given below:
 |
Some proteins are classified as fibrous and have evolved primarily for
structural functions. Examples of fibrous proteins would include ![]() -keratin,
found in hair and nails in exhibiting toughness appropriate for a protective
structure, silk (fibroin), collagen found in tendons and bone matrix and
elastin ( ligaments).
-keratin,
found in hair and nails in exhibiting toughness appropriate for a protective
structure, silk (fibroin), collagen found in tendons and bone matrix and
elastin ( ligaments).
Strength and/or elasticity are principal properties of fibrous proteins as is insolubility.
Insolubility occurs as a result of the presence of relatively "hydrophobic" amino acids located both in the interior and exterior protein domains.
Fibrous proteins typically have a single type of secondary structure.
For example, when considering
![]() -keratin
or collagen, simple helical structures define the proteins.
-keratin
or collagen, simple helical structures define the proteins.
For
![]() -keratin,
the helix is a right-handed alpha helix, whereas the collagen helix is
left-handed.
-keratin,
the helix is a right-handed alpha helix, whereas the collagen helix is
left-handed.
In accord with the requirement of insolubility, it is
not surprising that
![]() -keratin
has a significant percentage of "hydrophobic" residues,
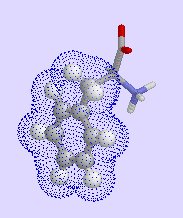
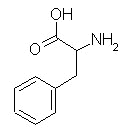
particularly phenylalanine,
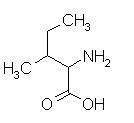
isoleucine,
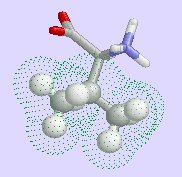
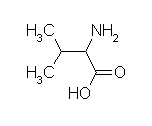
valine, methionine and
alanine.
-keratin
has a significant percentage of "hydrophobic" residues,
particularly phenylalanine,
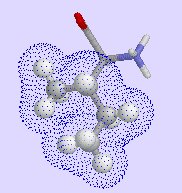
isoleucine,
valine, methionine and
alanine.
For collagen, constituent amino acids are also relatively hydrophobic consisting of glycine, alanine, proline and hydroxyproline.
Collagen is a little odd in that it is mostly composed of a repeating tri-peptide sequence glycine-X-proline or lysine-X-hydroxyproline.
This left-handed helical structure is characterized by three residues per turn.
While the insolubility property of these proteins has been appropriately described in terms of hydrophobic residues, the strength exhibited by these proteins is explained by particular structural associations.
In the case of collagen, three of the left-handed helical structures wrap around each other exhibiting a right-handed twist and forming a structure called tropocollagen.
These helical structures are called superhelical and exhibits substantial strength secondary to tight wrapping.
The tensile strength associated with collagen is thought to exceed steel wire of comparable cross-sectional area.
The strength of the structures is increased by the presence of cross-links which are covalent linkages between the chains. Disulfide bonds represent common cross-linking molecules (S--S).
 |
| phenylalanine | isoleucine | valine |
 |
 |
 |
 |
 |
 |
56attribution for these images from Chemistry 641 course by Professors Bahnson & Mueller