Opioids
"Narcotic" is a somewhat imprecise term because it suggests "narcosis", which is indicated of a somnolent state or "sleepy" state.
"Opioid analgesic" and therefore is a more appropriate term emphasizing the clinically important analgesic property which is the pharmacological property of importance in the therapeutic application of these agents.
Accordingly, opioids are used without the expectation that they themselves will cause sleep. However, opioids are frequently used in combination with anesthetics and in that context anesthesia may be obtained requiring less anesthetic.
All natural/semisynthetic opium alkaloid derivatives, synthetic agents, and other agents whose opioid-like effects are blocked by classical opioid antagonists, such as naloxone (Narcan) or naltrexone (ReVia).
Opium -- from the opium poppy (Papaver somniferum).
Opium is obtained following drying the milky juice from unripe seed pod.
|
|
Opium has a characteristic odor and bitter-taste with its chief active ingredient being morphine.
Also present are codeine, thebaine (a non-analgesic agent), noscapine and papaverine, a non-analgesic vasodilator.
Tincture of opium is called laudanum.
"Tincture" is a generic term which refers to an alcohol solution of a nonvolatile medicine. Paregoric is a mixture of opium, alcohol, and camphor.
An opioid full agonist activates opioid receptors, exhibiting high efficacy.
High efficacy refers to a maximal opioid effect, typically pain relief.
Full agonists may have comparable efficacies with the differing potencies meaning that different amounts of one drug compared to another may have to be given in order to achieve a maximal effect.
A partial agonist may itself cause agonist effects but because they can displace through competitive action a full agonist from its receptor, the net effect is a reduction in drug effect. As a result, a partial agonist, depending on circumstance, can act as either in agonist or an antagonist.
Antagonists: Pharmacological effects of opioids are mediated by interaction with differing opioids receptor types.
Most of the pharmacological effects as well as side effects, at least respiratory depression, are mediated by opioid-μ receptor interactions.
These agonist-mediated effects may be blocked by competitive inhibition by agents that occupy the the same receptor by do not activate it, yet prevent activation by agonists.
Furthermore, an opioid might be an agonist at one receptor subtype, but only a partial agonist or even in antagonist at another subtype.
Examples:
Naloxone (Narcan): pure antagonist: no effects normally associated with agonist binding.
Morphine: full agonist at μ receptor.
Codeine: partial or "weak" agonist indicating less than maximal theoretical effect despite complete receptor saturation.
Nalbuphine (Nubain) : agonist that one opioid receptor; antagonist at another.
Partial agonist/antagonist characteristics: replacement of methyl moiety on the nitrogen atom with larger substituents:
Allyl substitution-- nalorphine and naloxone
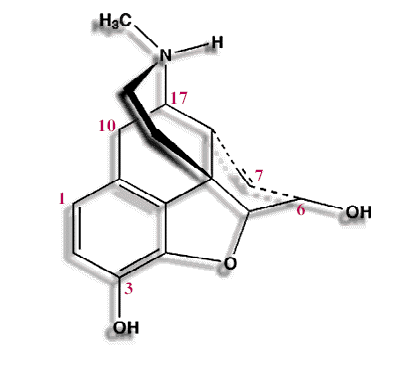
Substitutions at the C3 and C6 morphine hydroxyl groups (see below)
Pharmacokinetic properties altered
Methyl substitution at C3 reduces first-pass hepatic metabolism by glucuronide conjugation, as a consequence, codeine and oxycodone have a higher oral: parenteral potency
|
|
|
|
|
|
|
|
|
Acetylation of both morphine hydroxyls results in heroin which more rapidly crosses the blood-brain barrier compared morphine; in the brain heroin is rapidly hydrolyzed to monoacetylmorphine and morphine .
DISCLAIMER
|