Anesthesia Pharmacology: Physics and Chemistry for Anesthesiology
Gas Transport in Blood: Hemoglobin
The principal means by which blood is used to transport oxygen involves the specific binding protein hemoglobin. However in addition to this means, oxygen may be dissolved in blood in a manner proportional to its tension. Point A in the figure below represent a typical saturation point arterial blood and in the case of B, mixed venous blood (adapted from figure 11-1, reference 52).
|
|
The solubility coefficient for oxygen at about 38oC is as noted above, about 0.003 volume%/mm Hg (torr). Therefore, arterial blood with a PO2 of about 95 mm Hg (torr) would contain about 0.29 volume% of dissolved oxygen. If your oxygen is inhaled, alveolar PO to would increase towards 600 torr which would correspond to the dissolved oxygen concentration of about 2-volume%. The metabolic oxygen requirement at rest turns out to be approximately 6 volume% which can be reached in the context of a hyperbaric chamber pressurized to about 3 atm. 3 atm would correspond to an alveolar pressure of approximately 2000 mm Hg (torr). However, these high oxygen concentrations would be associated with oxygen toxicities thus limiting medical application to management of specific medical conditions.
An obvious question is whether or not dissolved oxygen in accord with the above relationship would be sufficient for the metabolic requirements of the body? As you might suppose, if we could get by without hemoglobin as an oxygen carrier we probably would-in a manner similar to the ice fish.
The graph above provides an indication of the capacity of blood to carry dissolved oxygen only. By examining the amount of oxygen extracted from the blood as a result of tissue metabolic needs, one obtains a difference, i.e. arteriovenous blood oxygen content difference of about 4 volume%. This number, 4 volume% needs to be compared with the difference in dissolved oxygen in arteriovenous blood, which is about 0.18 volume%. The ratio of 4 volume% to 0.18 volume% indicates that dissolved oxygen accounts for less than 5% of the total oxygen extracted by the tissues. This finding illustrates the central role of hemoglobin as an oxygen transport protein, accounting for greater than 95% of transport in the resting state and probably over 99% of oxygen transport during exercise.
![]() 53Given the importance of hemoglobin in the process, it is of
interest to know what the actual capacity is for hemoglobin to carry
oxygen.
53Given the importance of hemoglobin in the process, it is of
interest to know what the actual capacity is for hemoglobin to carry
oxygen.
It turns out that the blood if it were to contain 15 grams of hemoglobin/100 ml and given that each gram of hemoglobin has the capacity of about 1.34 ml of oxygen, then Hb can transport about 20 ml of oxygen per 100 ml of blood.
This analysis assumes essentially complete saturation of the protein by oxygen and this capacity is often referred to as 20 volumes%.
Whereas, the relationship between capacity and pressure (mm Hg; torr) for dissolved oxygen is linear, the linear relationship is not observed when hemoglobin as a carrier is analyzed.
The molecular basis for the deviation from linearity will be a subject of commentary later.
However, compare the figure below with that above and notice the difference in the shape of the curve for hemoglobin and the curve for oxygen solubility.
Moreover, the protein myoglobin is very similar to hemoglobin, but myoglobin contains only one polypeptide chain, whereas hemoglobin is composed of 4 separate subunits.
As we will see, the presence of multiple subunits is an important factor, really the determining factor that influences not only the shape of the hemoglobin saturation curve but also the underlying special properties concerning hemoglobin's ability to control oxygen availability.
|
|
illustration attribution: Raj Srinivasan, 1996
![]() 53Assuming 97% hemoglobin saturation, oxygen down hemoglobin
would correspond to about 19.4 ml/100 ml blood.
53Assuming 97% hemoglobin saturation, oxygen down hemoglobin
would correspond to about 19.4 ml/100 ml blood.
This quantity is reduced during blood flow through capillaries as oxygen exchanges with tissue, with the extent a reduction being about 5 ml (yielding about 14.4 ml oxygen which would correspond to about 75% hemoglobin saturation (PO2 of 40 mm Hg).
![]() The above condition, changes significantly during heavy exercise, since
interstitial fluid PO2 may fall to 15 mm Hg.
The above condition, changes significantly during heavy exercise, since
interstitial fluid PO2 may fall to 15 mm Hg.
In this circumstance less than 5 ml O2 remain associated with hemoglobin per 100 ml blood.
So in this case, about 15 ml/100 ml blood is actually delivered to the tissues (3X normal).
In addition to this change, one would take into account the significant increase in cardiac output associated with heavy exercise.
The combination of this cardiac output effect in the magnitude of O2 desaturation of hemoglobin would yield and up to a 20-fold increase in tissue oxygen transport. These argumentsgive rise to the concept of O2 utilization -- 25% at rest and nominally up to 75%-85% during exercise, with the possibility of achieving100% O2 utilization in cases of relatively low blood flow rates in combination with high local tissue metabolic requirements.
![]() Factors
influencing hemoglobin: oxygen transport
Factors
influencing hemoglobin: oxygen transport
Hemoglobin concentration effect:
Given that each g of hemoglobin has the capacity of about 1.36 ml O2 , the carrying capacity of blood will be dependent on the actual quantity of hemoglobin present.
This dependency is in addition to the O2 partial pressure effect.
Not surprisingly, anemia then would decrease O2 availability, potentially to an extent that would induce as a physiological responses.
Atmospheric pressure effect: Consider the sigmoidal shape of the oxygen-saturation curve below
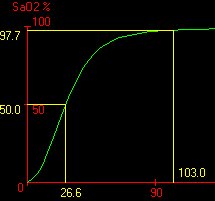
If we were to consider that the normal PO2 is about 103 mm Hg, there would be an interest in seeing how Hb saturation would be affected at lower pO2 levels, such does might occur in a higher elevations.
Inspection of the graph above indicates that a drop from 103 mm Hg to about 60 mm Hg would correspond to only a slight drop in Hb saturation, maybe to about 90%.
In that case, it would be still relatively easy for the tissues to remove the required 5 ml of O2 per 100 ml of blood-the typical requirement.
The other extreme would apply in a "hyperbaric" case with very high alveolar PO2 levels.
Hypothetically, if the alveolar PO2 were 500 mm Hg, hemoglobin would essentially remain at the same (really nearly the same) saturation level, i.e. saturation cannot exceed 100%.
In conclusion, even though alveolar O2 concentrations may vary significantly (60 mm Hg-> 500 mm Hg), the tissue-PO2 is held relatively constant as a result of the particular biochemical characteristics of hemoglobin reflected in the unusual PO2 vs. Hb saturation relationship (curve).
![]() Several factors influence the position of the
oxygen-hemoglobin saturation curves-some factors resulting in a shift to
the right and others in a shift to the left. For example, if the
pH decreases from 7.4 to 7.2 there is about a 15% shift in the
dissociation curve to the right (see below).
Several factors influence the position of the
oxygen-hemoglobin saturation curves-some factors resulting in a shift to
the right and others in a shift to the left. For example, if the
pH decreases from 7.4 to 7.2 there is about a 15% shift in the
dissociation curve to the right (see below).
An increase in the pH, i.e. more alkaline, shifts the dissociation curve to the left --the description of these curve as "dissociation curves" reflects the underlying process of oxygen combining with hemoglobin and then (dissociation) unbinding with hemoglobin. This dissociation and association process is highly dynamic and is influenced by changing tendencies of oxygen to remain bound.
Other factors that tend to shift the dissociation curve to the right to include:
(a) an increase in carbon dioxide concentration,
(b) increased blood temperature and
(c) an increase in a metabolically important phosphate-compound,2,3 diphosphoglycerate (DPG, DPG).
Effect of pH on the position of the oxygen-hemoglobin saturation curve
As blood returns to the lung, carbon dioxide dissociates from Hb and enters the alveoli. This process is associated with a reduction in blood PCO2 with an attendant decrease in [H+] due to the decrease in blood carbonic acid. This process results in a shift of the curve to left and up. A leftward and upward shift, if we examine the curve, below,results in a higher degree of hemoglobin saturation and any given oxygen tension. This increased saturation creates conditions favoring oxygen transport to the tissues.
The tissue, as a result of metabolism, is producing carbon dioxide and therefore the blood, now at the capillary level, is exposed to higher levels of carbon dioxide (increasing blood carbonic acid in decreasing pH). This condition causes a shift to the right in the hemoglobin-oxygen saturation curve which means that there will be reduced hemoglobin saturation in any given oxygen partial pressure. Reduced hemoglobin saturation means that more oxygen has dissociated at that particular partial pressure we chose, thus making more oxygen molecules available to the tissue.
|
|
The underlying mechanism for these curve shifts is based on the presence of titratable groups in Hb which can accept or release the proton (H+). The consequence of accepting a proton (or releasing one) is that the entire structure of hemoglobin may be somewhat altered and a consequence of this change is a change in the likelihood (affinity) of oxygen binding. The dependency of the curve shape on pH is referred to as the Bohr effect, named after Christian Bohr, a Dutch physiologist who first noted the effect of pH on oxygen association from hemoglobin.
At this point, it may be useful to comment a little further about the molecular basis for the oxygen-hemoglobin dissociation curve.
The reason that the shape of the curve is different from the myoglobin, simple hyperbolic shape, is that there are progressive changes in the affinity of hemoglobin for oxygen has a function of saturation.
This analysis suggests that whereas the binding of the first oxygen molecule the hemoglobin might be associated with a relatively lower affinity, as a consequence both the first binding, subsequent binding steps occur more readily.
That is, with increasing oxygen saturation, there is a progressive increase in a tendency of the second, third, and ultimately fourth oxygen molecule to bind.
Inspection of the curve itself suggests that the increase in saturation occurs initially relatively slowly and then as the oxygen concentration continues to climb the rate of change in Hb-oxygen saturation increases rather rapidly.
The portion of the curve associated with the most rapid increase in slope covers a concentration range associated with the difference between working and resting muscle oxygen tension.
The structural basis of the sigmoidal curve shape is the presence of 4 interacting subunits that comprise hemoglobin.
A structural change associated with O binding and a first site induces changes in the binding domain of other subunits-in this case in the direction of increasing the likelihood of oxygen binding.
Proteins that exhibit the sort of property are generally referred to as exhibiting cooperativity and furthermore the type of cooperativity associated in this case is positive cooperativity.
Positive cooperativity refers to the process by which sequential saturation of binding sites is associated with increasing affinities-that is, binding to the first site makes binding to the second site easier, etc.
Each subunit consists of a bound heme.
Hemoglobin consists of two α subunits and two β subunits.
The iron, part of a porphyrin structure, defines the oxygen molecule binding site.
Formally, the heme group is an example of a "prosthetic group" which in this case is a herterocyclic ring containing 4 pyrrole rings with Fe2+ as the central atom (for hemoglobin).
The central atom is Fe3+ for cytochomes (liver oxidizing enzymes) and Mg2+ for chlorophyll.
|
|
|
The heme group is a molecular template which consists of protoporphyrin (above) to which Fe2+ (ferrous iron form) is bound.
Protoporphyrin consists of four 5-membered heterocyclic rings (pyrroles) bonded together to form a tetrapyrrole macrocycle.
The ferrous iron form exhibits 6 bonding possibilities, for of which resid in the plane of the flat porphyrin molecule (coplanar); two bonds are perpendicular, one of which associates with a nitrogen of the critical histidine residue described below-the other bond is available for oxygen association.
Of the many amino acids that make up hemoglobin, there is one special amino acid, a proximal histidine (His) which is particularly important to the mechanism of positive cooperativity.
When O2 binds to Fe, there is physical movement of the histidine residue (α chain 87;β chain:His92) and its helix which in turn adjusts relative relationship of alpha and beta subunits.
There is a resultant change in the Fe conformation of the sites which remain free (no O2 bound)
One additional point has to do with the influence of protons (H+) on the binding curve position, i.e. displacement of the curve to the left or right.
This phenomenon classifies hemoglobin as an example of an "allosteric" protein in which its oxygen affinity is affected by a separate, effector molecule.
In this example, the proton is the effector; however, other well-known effectors include CO2 and organic phosphates, especially 2,3 diphosphoglycerate (DPG).
Allosteric proteins have independent binding sites for effectors which are separate from either their catalytic site in the case of an enzyme or a critical binding site.
|
|
|
2,3 diphosphoglycerate (DPG): Organic phosphates have a significant effect on the oxygenation curve for hemoglobin. Probably the most significant molecule physiologically is 2,3 diphosphoglycerate (DPG).
2,3 diphosphoglycerate (DPG)
The effect of DPG follows from its binding preferentially to deoxyhemoglobin. Accordingly, DPG will shift the oxygenation curve in favor of the deoxy state. In order to saturate about 50% of the available DPG binding sites on hemoglobin, a DPG concentration of about 15 micromolar is required. Since normally the erythrocyte concentration of DPG is many orders of magnitude higher (4 mM), the deoxy form is strongly favored.
Typically, physiological levels of DPG move the oxygen-hemoglobin association curve somewhat to the right; however, this effect is more pronounced during somewhat prolonged hypoxic states during which DPG concentrations increased, shifting the curve further to the right.
The consequence of this curve shift is that oxygen may be released to the tissue at as much as 10mm Hg elevated tissue pO2 than without the DPG effect. It has been suggested that adaptation to hypoxic conditions (including altitude acclimatization) depends in part on alterations in DPG levels.
52 Slonim, NB and Hamilton, LH "Blood-Gas Transport" in Respirtory Physiology 5th Edition, The C.V. Mosby Co., St. Louis, MO, 1987, pp. 135-153.
53Guyton, AC and Hall, J.E. "Transport of Oxygen and Carbon Dioxide in the Blood and Body Fluids" in Textbook of Medical Physiology, 10th edition,WB Saunders Company, Philadelphia, pp. 463-473, 2000.