Medical Pharmacology Chapter 35 Antibacterial Drugs
Penicillins And Others
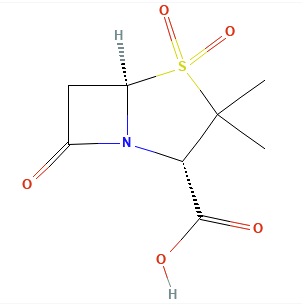
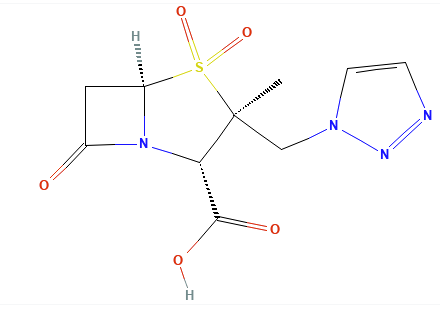
Introduction: Beta-Lactamase Inhibitors: Sulbactam, Tazobactam, Avibactam, Relebactam, Clavulanic Acid.
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
![]() Beta-lactamase inhibitors (BLIs) are a class of drugs
co-administered with beta-lactam antibiotics to combat bacterial
resistance.
Beta-lactamase inhibitors (BLIs) are a class of drugs
co-administered with beta-lactam antibiotics to combat bacterial
resistance.
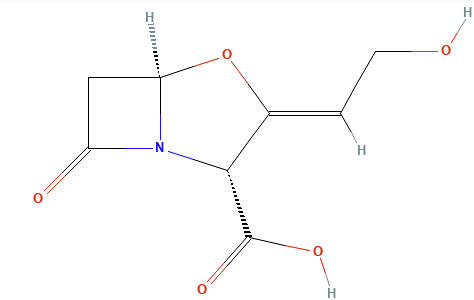
The first generation of clinically successful beta-lactamase inhibitors are beta-Lactam -containing molecules.1
The Beta-lactamase inhibitor, because of its structural similarity to penicillin, binds to a serine residue at the active site of a class A or D beta-lactamase.
The enzyme acts on the inhibitor as it would a normal substrate, leading to the formation of the covalent acyl-enzyme intermediate.
Because of the formation of this intermediate, the substrate is sometimes referred to as the hemi-substrate.
The acyl enzyme is unstable and undergoes internal chemical rearrangements resulting in a new, highly reactive species that forms a second, and very stable covalent bond with another residue in the active site.
![]() This
process involves cross-linking of structures at the active
center and permanently inactivates the inhibitor.11
This
process involves cross-linking of structures at the active
center and permanently inactivates the inhibitor.11
This mechanism is associated with the first generation agents.
There mechanism of action is through neutralizing beta-lactamase enzymes produced by many bacteria, thereby protecting the companion antibiotic’s beta-lactam ring from degradation.1
The five inhibitors discussed here, clavulanic acid, sulbactam, tazobactam, avibactam, and relebactam each enhance the spectrum and efficacy of their associated antibiotics.
![]() The β-lactam class of antibiotics, which includes penicillins,
cephalosporins, monobactams, and carbapenems, represents the
cornerstone of antibacterial therapy and is among the most
consequential drug classes in medical history.2
The β-lactam class of antibiotics, which includes penicillins,
cephalosporins, monobactams, and carbapenems, represents the
cornerstone of antibacterial therapy and is among the most
consequential drug classes in medical history.2
Since the discovery of penicillin, these agents have become the
most widely prescribed antibiotics globally, accounting for a
substantial percentage (≈65%) of all antibiotic usage due to their
potent bactericidal activity, broad spectrum, and generally
favorable safety profile.
 |
|
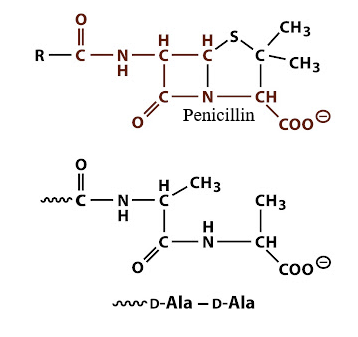
This structural similarity allows them to bind covalently to and inactivate essential enzymes known as penicillin-binding proteins (PBPs).
PBPs are responsible for the final steps of peptidoglycan synthesis, the rigid polymer that provides structural integrity to the bacterial cell wall.
By inhibiting PBP-mediated cross-linking, β-lactams disrupt cell wall maintenance and synthesis, leading to cell lysis and bacterial death.2,3
Diversity of β-lactamases, with thousands of variants identified, required a systematic classification scheme to understand their function and predict their clinical impact.
The most widely used system is the Ambler classification,
proposed by Richard Ambler in 1980, which groups these enzymes
into four major molecular classes (A, B, C, and D) based on
their amino acid sequence homology.
This molecular framework aligns closely with the functional properties of the enzymes.
Classes A, C, and D:
These are known as serine β-lactamases because they utilize a
critical serine residue in their active site to initiate
hydrolysis of the β-lactam ring.
Class A8
This class includes the common TEM-1, TEM-2 and HV-1 penicillinases in addition to the extended-spectrum ß-lactamases (ESBLs) like the CTX-M family which can degrade third-generation cephalosporins.
This class also includes Klebsiella pneumoniae carbapenemases (KPCs) which inactivate carbapenems.
Class C8
This class consist of AmpC cephalosporinases, which are often
chromosomally encoded and can be induced or overexpressed to
confer resistance to most cephalosporins.
Members of this class are metallo-β-lactamases (MBLs), mechanistically distinct from the serine-based enzymes.
These enzymes require one or two divalent zinc ions as cofactors
in their active site for catalysis.
MBLs, such as NDM, VIM, and IMP types, possess an exceptionally broad hydrolysis spectrum, capable of inactivating nearly all β-lactam antibiotics, including carbapenems.
The monobactam aztreonam is an exception
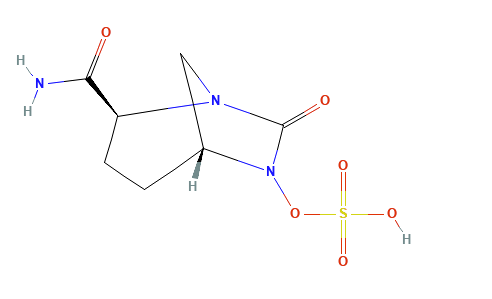
Clavulanic acid, sulbactam, and tazobactam all have a beta-lactam core and act as classic “suicide inhibitors.”
The process involves two steps: (1) acylation of the beta-lactamase active site followed by a secondary reaction (2) that permanently inactivates the enzyme.1,11
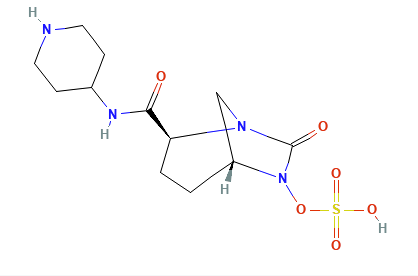
The newer diazabicyclooctane inhibitors, avibactam and relebactam, do not have a beta-lactam core and act as reversible, high-affinity substrates.
Instead of the Beta-Lactam core, these agents feature a diazabicyclooctane (DBO) core.
The newer compounds act as reversible covalent inhibitors.
These molecules also isolate the active site serine of at target ß-lactamase inactivating the enzyme initially.
Since the reaction is reversible,a deacylation reaction occurs generating the original, fully active inhibitor which can then inhibit another beta-lactamase molecule.
These newer inhibitors also acylate the enzyme but, upon deacylation, the intact inhibitor is regenerated rather than destroyed (one step).1,11,13
This reversible covalent binding allows avibactam and relebactam to inactivate multiple enzyme molecules sequentially, making them highly effective against a broad range of serine beta-lactamases.11
All of these inhibitors are effective against Ambler class A beta-lactamases (e.g. common penicillinases and many extended-spectrum beta-lactamases, ESBLs), and most also inhibit class C (AmpC) enzymes.11
Avibactam and relebactam additionally have some activity against class D enzymes (such as OXA-48 carbapenemases), though not against class B metallo-beta-lactamases.11
![]() By
neutralizing these enzymes, BLIs restore the activity of their
partner beta-lactam antibiotics against otherwise resistant
bacteria.
By
neutralizing these enzymes, BLIs restore the activity of their
partner beta-lactam antibiotics against otherwise resistant
bacteria.
August, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |