Medical Pharmacology Chapter 35 Antibacterial Drugs
Penicillins And Others
Beta-lactamase inhibitors
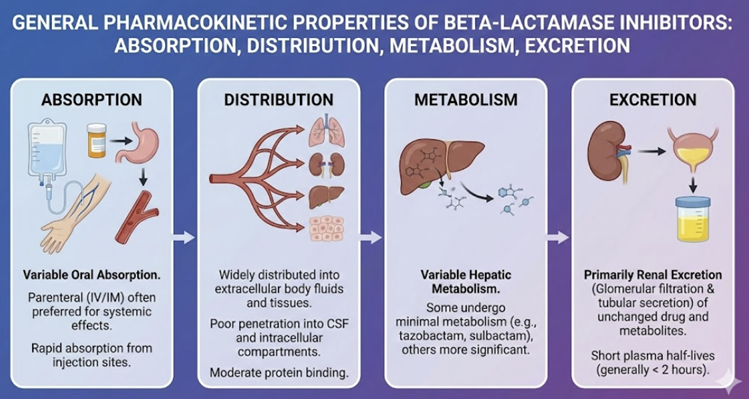 |
Pharmacokinetic Properties (ADME)
Absorption
Among these inhibitors, only clavulanic acid is routinely used orally (as amoxicillin/clavulanate).
Clavulanate is moderately well-absorbed orally, with a bioavailability around 60–70% for immediate-release formulations, and is often given with food to improve tolerability.1
Sulbactam is not orally absorbed in its active form (it is given parenterally), but an oral prodrug (sultamicillin) exists, which is hydrolyzed after absorption to yield sulbactam plus ampicillin.3
Tazobactam (i.v. or i.m), avibactam (ceftazidime-avibactam, i.v.)4, and relebactam (e.g. Recarbrio™, consisting of imipenem, cilastatin and relebactam) by IV infusion)5 are not available orally and are administered parenterally along with their beta-lactam partners.
Distribution
These inhibitors distribute well into body fluids and tissues, similar to their companion antibiotics.
Clavulanic acid and amoxicillin have similar volumes of distribution and short half-lives (~1 hour).1,6
Sulbactam’s volume of distribution is about 12 liters, indicating it is primarily extracellular; it is ~38% protein bound.1
Relebactam’s protein binding is ~22%8, and it penetrates into tissues like lung epithelial lining fluid at therapeutically relevant levels.7
Metabolism
Most beta-lactamase inhibitors undergo minimal metabolism.
Clavulanic acid is partially metabolized in the liver (a portion of the dose is not recovered unchanged in urine), whereas sulbactam, tazobactam, avibactam, and relebactam are largely excreted unchanged.
Sulbactam has <25% hepatic metabolism with minimal biliary excretion9, and tazobactam similarly has only minor metabolism (one inactive metabolite).2
Avibactam10 and relebactam8 are not significantly metabolized by the liver.
Excretion
Renal excretion is the primary elimination route for all these agents.
They are filtered and secreted by the kidneys, often via active transport mechanisms.
Clavulanic acid has a half-life of ~0.8 hours and ~37–57% of an oral dose is excreted unchanged in urine within 6 hours.1
Sulbactam has a half-life ~1 hour, with ~75% of a dose excreted unchanged in urine.1
Tazobactam likewise has ~1 hour half-life; about 57–64% is recovered unchanged in urine over 24 hours.1
Avibactam has a slightly longer half-life (~2 hours); roughly 85–97% of an IV dose is excreted unchanged in the urine, with over half recovered in the first 2 hours.4,11
Relebactam has a half-life of ~1–1.5 hours and >90% is eliminated unchanged by the kidneyss.12,13
![]() Because renal clearance is so important, doses of these
inhibitors (and their partner antibiotics) must be reduced in
patients with renal impairment to prevent accumulation.2
Because renal clearance is so important, doses of these
inhibitors (and their partner antibiotics) must be reduced in
patients with renal impairment to prevent accumulation.2
Probenecid, which blocks renal tubular secretion, can raise levels of beta-lactamase inhibitors (e.g. it decreases avibactam’s renal clearance by ~56–70%), so concomitant use of probenecid is generally not recommended.
Probenecid inhibits avibactam uptake because probenecid is a potent Organic Ion Transporter (OAT) inhibitor.
Probenecid in combination with avibactam would potentially reduce avibactam elimination.
For example, coadministration of ceftazidime/avibactam with probenecid would not be recommended.14
August, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |