Medical Pharmacology Chapter 35 Antibacterial Drugs
 |
 |
 |
 |
|
Sulfonamides: Audio Overview |
Sulfonamides (sulfa drugs) are a class of synthetic antimicrobial agents that marked the beginning of modern antibiotic therapy in the 1930s.1
These were the first effective systemic antibacterial drugs, derived from the red dye prontosil (sulfanilamide), and these agents represented as dramatic improvement in treatment of infections prior to the availability of penicillins.
Sulfonamides are analogues of p-aminobenzoic acid (PABA), a substrate bacteria require for folic acid synthesis.1
By mimicking PABA, sulfonamides inhibit folate synthesis in microbes.2
Although their usage has declined due to widespread resistance and the availability of other antibiotics, sulfonamides – particularly in combination with trimethoprim – remain important agents for certain infections.
 |
%20image1.png) |
%20image1.png) |
| Generic tertiary sulfonamide | Sulfamethazine (SMZ) | Sulfadiazine (SDZ) |
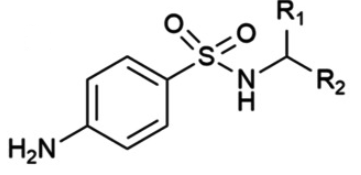
All antimicrobial sulfonamides are synthetic derivatives of the parent compound, sulfanilamide.
Sulfonamides are chemically defined by a specific organosulfur functional group with the general structure R-S(=O)2 , where a sulfonyl group (O=S=O) is directly linked to an amine group (-NH2).
This core structure is relatively rigid, a property that
contributes to the crystalline nature of these compounds.
Pharmacological and physical characteristics of individual sulfonamide agents are determined by the various chemical substitutions made on this fundamental nucleus.3
Distinguishing between antimicrobial sulfonamides and non-antibiotic drugs that also contain a sulfonamide functional group, such as certain diuretics (e.g., hydrochlorothiazide), hypoglycemic agents (e.g., sulfonylureas), and anti-inflammatory drugs (e.g., celecoxib) is important.
Key structural feature implicated in many of the severe hypersensitivity reactions to sulfonamide antibiotics is the arylamine group at the N4 position of the benzene ring.
This group is absent in most non-antibiotic sulfonamides, meaning that a history of allergy to a sulfonamide antibiotic does not typically predict an allergic reaction to a non-antibiotic sulfonamide.3
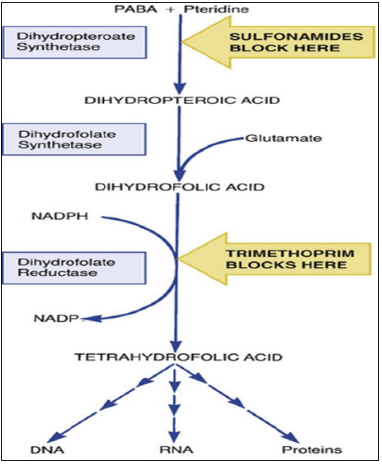
Sulfonamide Mechanism of Action
 |
|
![]() Sulfonamides
are bacteriostatic
agents that inhibit bacterial growth by interfering with folic
acid synthesis.
Sulfonamides
are bacteriostatic
agents that inhibit bacterial growth by interfering with folic
acid synthesis.
The bacteriostatic nature implies that adequate host immune function is important for clearing the infection after sulfonamides suppress bacterial proliferation.
As structural analogues of PABA, these agents and competitively inhibit dihydropteroate synthase (DHPS), which normally converts PABA into dihydrofolic acid.5,1
This enzyme catalyzes the condensation of 6-hydroxymethyl-7,8-dihydropterin pyrophosphate with para-aminobenzoic acid (PABA) to form dihydropteroate, an essential precursor for bacterial DNA synthesis.
As structural analogs of PABA, sulfonamides compete for the enzyme's active site with higher affinity than the natural substrate, effectively blocking bacterial folate production.7
By blocking this first step in folate production, sulfonamides prevent bacteria from synthesizing folic acid cofactors needed for DNA and RNA building blocks, thereby halting cell growth and division. 2 Human cells are not harmed by sulfonamides as folic acid synthesis inhibitors because mammals do not synthesize folic acid from PABA. Therefore, the selective toxicity stems from a fundamental difference in folate metabolism: bacteria must synthesize folate de novo, while humans obtain it through dietary sources.6
This biochemical distinction allows sulfonamides to kill bacteria without significantly affecting human cells, providing an excellent therapeutic index when used appropriately.
To summarize then:
1. Bacteria that cannot utilize preformed folate must synthesize it de novo from PABA.
2. Sulfonamides, as structural analogues of PABA, act as competitive inhibitors of the bacterial enzyme dihydropteroate synthase (DHPS).8
3. By occupying the active site of DHPS, sulfonamides block the incorporation of PABA into dihydropteroic acid.
This action prevents the synthesis of dihydrofolic acid and,
subsequently, its active form, tetrahydrofolic acid (THF).
THF depletion halts bacterial DNA replication and protein
synthesis, leading to the cessation of growth
![]()
By
inhibiting successive steps in the pathway (sulfonamides block
dihydropteroate synthase and trimethoprim or pyrimethamine block
dihydrofolate reductase – see figure above), the drugs together
prevent the conversion of PABA to
tetrahydrofolate,
which is the active folate form required for synthesizing
nucleic acids
Inhibition associated with this two-drug combination leads to a
maximal antibacterial
effect that is often bactericidal, even though each
agent alone is bacteriostatic
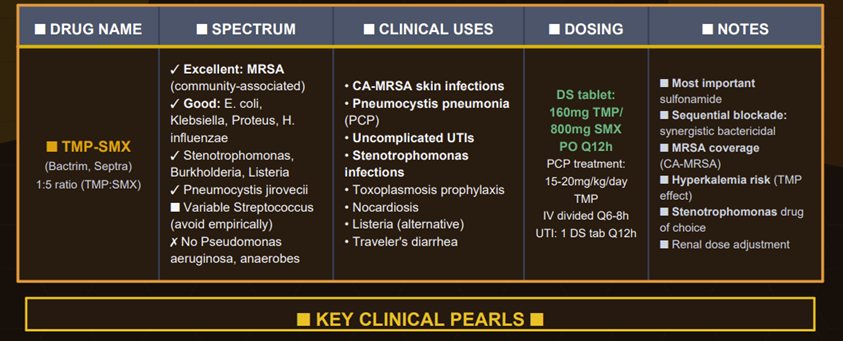
![]() Clinically,
TMP-SMX is a broad-spectrum, bactericidal combination used for both
treatment and prophylaxis of various infections (detailed
under Therapeutic Uses).
Clinically,
TMP-SMX is a broad-spectrum, bactericidal combination used for both
treatment and prophylaxis of various infections (detailed
under Therapeutic Uses).
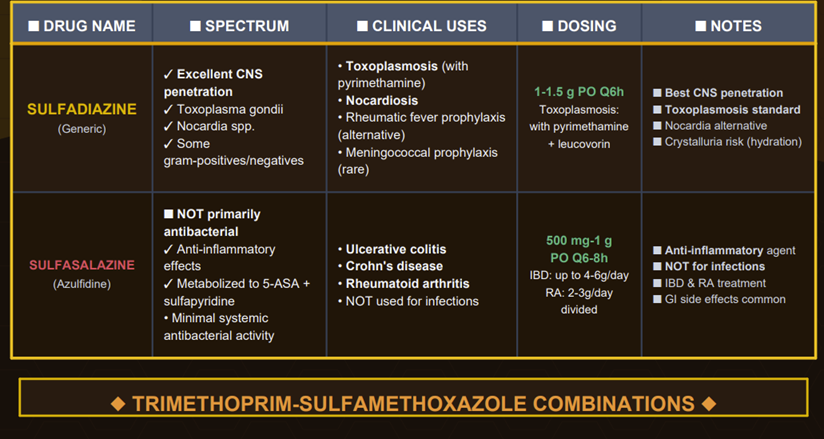
Another example of synergism is sulfadiazine + pyrimethamine, which is the first-line therapy for toxoplasmosis, leveraging the same sequential folate inhibition principle in protozoa.
This combination (plus leucovorin rescue) is highly effective at
treating Toxoplasma encephalitis
 |
||||
|
![]()
In
combination with trimethoprim (TMP-SMX), the spectrum
is enhanced and often bactericidal, covering organisms such as:
Group A Streptococcus (S. pyogenes) is not reliably killed by sulfonamides18.
TMP-SMX is not recommended group A streptococcal pharyngitis, (a.k.a. strep throat)as it fails to eradicate the bacteria or prevent rheumatic fever complications
Enterobacterales (e.g. E. coli, Klebsiella, Enterobacter, Proteus, Shigella, Salmonella): variable activity with many resistant.19
TMP-SMX is active against many E. coli (hence its use in UTIs), Shigella, and some Salmonella, but resistance in these families is now widespread.20
Haemophilus influenzae may be susceptible. In one report the rate of susceptibility was 36.4%.21
Neisseria species – many strains of N. meningitidis and N. gonorrhoeae were sulfonamide-susceptible in the past, but resistance is now high, limiting use. (Sulfonamides are no longer recommended for gonorrhea, and meningococcal prophylaxis with sulfa has been replaced by other agents due to resistance.)22,23
Anaerobic bacteria are not inhibited by sulfonamides to a useful extent.19
Pseudomonas aeruginosa is intrinsically resistant.19
Enterococci are also generally resistant because they can utilize exogenous folate and bypass the sulfonamide mechanism.19
Treponema pallidum (syphilis) is not treated by sulfonamides.19
In summary, the in vitro spectrum is broad, but actual clinical utility is narrower today due to resistance.
![]() Bacterial
Resistance Mechanisms
Bacterial
Resistance Mechanisms
 |
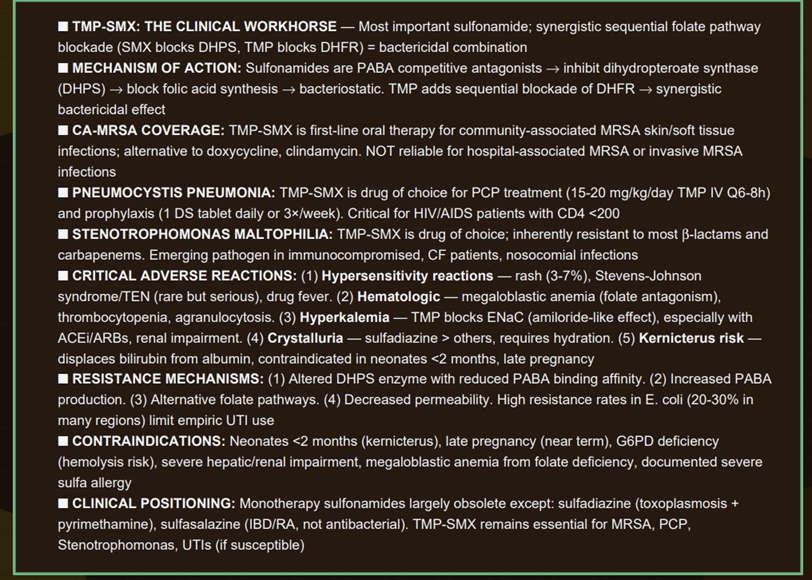
Resistance to sulfonamides is widespread and has markedly limited their stand-alone use.
After extensive of sulfonamide use in both humans and agriculture, many bacterial strains have acquired one or more resistance mechanisms.
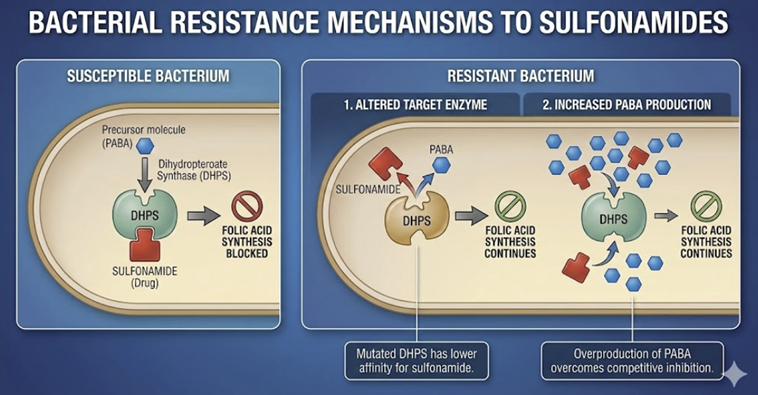
![]() Key
sulfonamide resistance mechanisms include:
Key
sulfonamide resistance mechanisms include:
Altered target enzyme: Bacteria can acquire mutations in the gene for dihydropteroate synthase (folP gene), producing an enzyme with lower affinity for sulfonamides.
This common mechanism (e.g. seen in E. coli and other Enterobacterales) may be plasmid-mediated.
Some Neisseria spp. have even acquired new folP gene variants via horizontal transfer, raising concern for broader dissemination of resistance.24,25
Overproduction of PABA (Para-amino benzoic acid): By synthesizing an excess of the natural substrate PABA, the bacteria can out-compete the drug at the enzyme site.26
This mechanism has been noted in resistant Staphylococcus aureus and certain Neisseria gonorrhoeae strains, among others.27
The high PABA levels make it more likely that the natural substrate will bind instead of the competing sulfonamide.
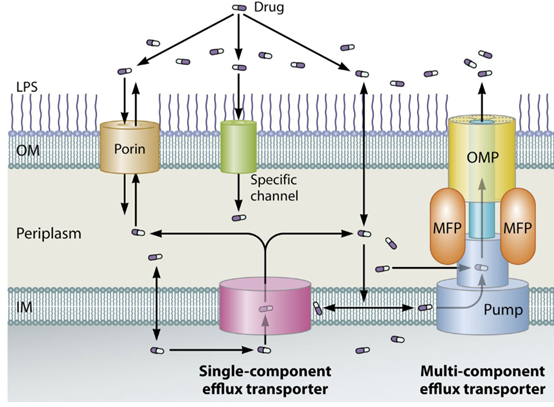
Decreased permeability or active efflux: Some bacteria reduce uptake of sulfonamide or increase efflux pumps to remove it, lowering the intracellular drug concentration.
This mechanism is less specific but contributes to resistance in various Gram-negative organisms.28
 |
|
Alternative metabolic pathway: A less common mechanism is bacteria acquiring the ability to use exogenous folic acid or an alternative pathway for folate metabolism, rendering them less dependent on the PABA → folate synthesis route. (For example, enterococci inherently use preformed folate and thus are intrinsically resistant to sulfonamides.)4
Plasmid-mediated resistance genes: Plasmids can carry genes encoding a drug-resistant dihydropteroate synthase enzyme.
Such plasmids (e.g. the sul genes like sul1, sul2, sul3) are now prevalent in many E. coli and other Gram-negatives, often alongside other resistance genes.
These plasmid-borne enzymes function normally but do not bind sulfonamides effectively.
Plasmid transfer between bacteria (even across species) has accelerated the spread of sulfonamide resistance.25
More about Resistance and Sulfonamides:
The prevalence of sulfonamide resistance, particularly among Gram-negative pathogens like E. coli, is a major clinical challenge.
Resistance rates frequently exceed the
20% threshold at which the Infectious
Diseases Society of America (IDSA)
recommends against the empiric use of
TMP/SMX for uncomplicated urinary tract
infections.
Surveillance data reveal significant global and regional variation.
For example, a 2020 meta-analysis in
Iran reported a 62% resistance rate
for E. coli to cotrimoxazole31,
while 2023 data from Wales showed a
33.8% resistance rate in E. coli
bloodstream isolates.
The World Health Organization (WHO) noted that in 2020, approximately one in five urinary tract infections caused by E. coli globally demonstrated reduced susceptibility to cotrimoxazole.29
A critical factor driving the persistence of high-level sulfonamide resistance is the phenomenon of genetic linkage and co-selection.
The sul genes are often located
on the same mobile genetic elements
(plasmids and integrons) that carry
genes conferring resistance to other
antibiotic classes, such as
tetracyclines (tet genes) and
aminoglycosides (strA/B for
streptomycin).
This dynamic helps explain why
resistance rates have remained
stubbornly high for decades, even in
regions where sulfonamide consumption
has declined
July, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |