Medical Pharmacology Chapter 35 Antibacterial Drugs
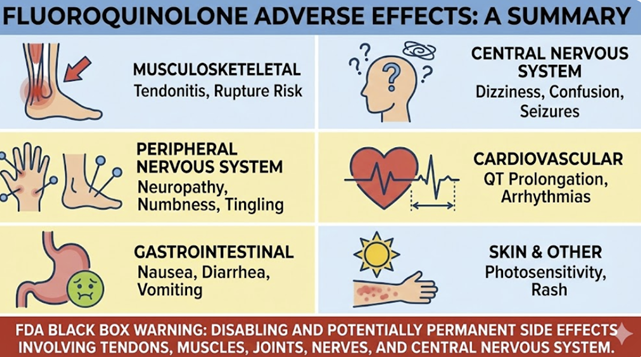 |
Gastrointestinal
Nausea, vomiting, diarrhea are the most frequent side effects (occurring in a few percent of patients).1,2
These are usually mild and self-limited.
Fluoroquinolones can also cause abdominal pain or dyspepsia in some cases
Clostridioides difficile colitis has been associated with broad-spectrum antibiotic use, including fluoroquinolones, so any patient developing significant diarrhea after use should be evaluated for C. difficile infection.1,3
Central Nervous System1
Quinolones can penetrate the CNS to some extent and may cause headache, dizziness, insomnia, or mood changes.4
Elderly patients, in particular, have reported confusion, agitation, or even hallucinations while on fluoroquinolones.5
Fluoroquinolones can induce seizures in patients of advanced age and frailty as described in this case report.6
These CNS effects may be related to quinolone-induced GABA receptor antagonism in the brain.7
The FDA has warned of potentially permanent neurologic effects, including peripheral neuropathy (tingling, nerve pain that can last long after the drug is stopped).
Should a patient report new neuropathic symptoms such as burning, numbness, weakness, the drug should be discontinued to prevent possible long-term nerve damage.8
Tendinopathy and Musculoskeletal
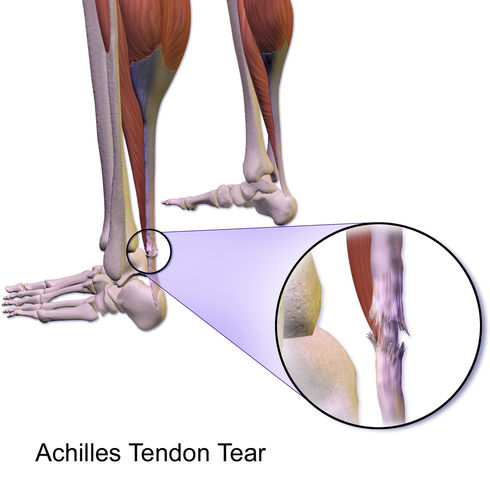
A serious adverse effect of fluoroquinolones is tendinitis and tendon rupture.
 |
|
Fluoroquinolones can cause degeneration of collagen in tendons.9
The Achilles tendon is classically affected, but rotator cuff tendons can be involved.10
Tendonitis can occur within days of starting therapy or even months after completion.11
Tendon rupture, particularly of the Achilles, has been documented, sometimes requiring surgical repair.11
The risk is higher in older patients (over 60), in those on concurrent corticosteroids, and in organ transplant recipients.11
There appears to be an increased likelihood of aortic aneurysm formation in adults following fluroquinolone administration.12,13
Several fluoroquinolones can prolong the cardiac QT interval on ECG, which in turn can precipitate a type of arrhythmia called torsades de pointes (a potentially fatal ventricular arrhythmia).14
This effect is most pronounced with sparfloxacin, grepafloxacin, moxifloxacin, and to a lesser extent gemifloxacin.
![]() In
1999, grepafloxacin was withdrawn due to QT-related
fatalities, and sparfloxacin was also later removed from the
market.
In
1999, grepafloxacin was withdrawn due to QT-related
fatalities, and sparfloxacin was also later removed from the
market.
Levofloxacin and ciprofloxacin appear to have minimal effects on the QT interval.15,16,17
Moxifloxacin remains available but carries a QT warning; it should not be used with other QT-prolonging drugs like certain antiarrhythmics, tricyclic antidepressants, macrolide antibiotics, etc.18
Patients at baseline risk for QT prolongation (those with long QT syndrome, on class IA/III antiarrhythmics, or with electrolyte imbalances) should generally avoid the quinolones that prolong QT.19
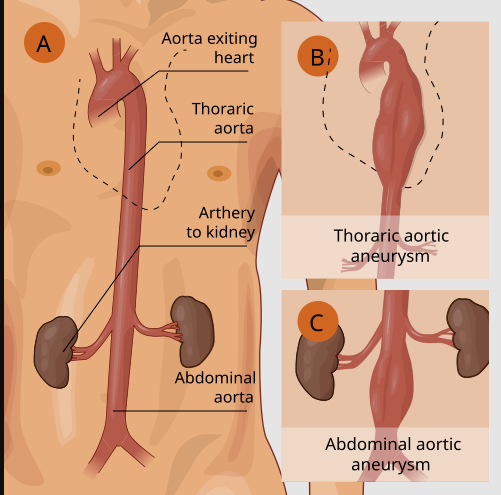
Aortic Aneurysm/Dissection
 |
|
![]() There appears to be an increased likelihood of aortic
aneurysm formation in adults following fluroquinolone
administration.12,13
There appears to be an increased likelihood of aortic
aneurysm formation in adults following fluroquinolone
administration.12,13
An emerging safety concern is a link between fluoroquinolone use and aortic aneurysm or dissection.
Observational studies and case reports prompted the FDA in 2018 to issue a warning that fluoroquinolone antibiotics can increase the risk of aortic aneurysm rupture or dissection,13 especially in susceptible individuals. Patients with existing aortic aneurysm, Marfan syndrome, Ehlers-Danlos, or elderly with atherosclerosis are thought to be at higher risk.20
Fluoroquinolones should likely be avoided in patients with known aortic aneurysms or significant risk factors for aneurysm if an alternative antibiotic is available.
Phototoxicity
Some quinolones can cause photosensitivity, meaning the skin is more prone to sunburn or phototoxic reactions when exposed to UV light.
Among more modern fluoroquinolones ciprofloxacin and levofloxacin have lower rates but can still cause sun reactions in a small percentage of patients, so reasonable sun precautions are advised.22 Lomefloxacin appears the most phototoxic fluorquinolone with moxifloxacin the least phototoxic.21
Fluoroquinolones have been linked to disturbances in blood glucose.
Fluoroquinolones can affect glucose regulation, especially in diabetic patients on hypoglycemic agents.23
For instance, moxifloxacin and levofloxacin have had reports of hypoglycemia (or hyperglycemia) in diabetics.
In 2018, the FDA highlighted that fluoroquinolones can lead to serious hypoglycemia, sometimes resulting in coma.24
They concurrently noted mental status side effects (mentioned above).
Diabetic patients on insulin or sulfonylureas should be carefully monitored if a fluoroquinolone is necessary.25
Ciprofloxacin in combination with glyburide (a sulfonylurea) has precipitated hypoglycemic reactions, so caution is warranted.26
Other
Fluoroquinolones can exacerbate myasthenia gravis.27
All systemic fluoroquinolones carry a boxed warning noting worsening of myasthenia gravis associated with fluorquinolone administration.28
They have a neuromuscular blocking effect that can precipitate more severe muscle weakness or respiratory difficulty in MG patients.29
Chelation with Cations
Concomitant administration of quinolones with antacids (containing Mg2+ or Al3+), sucralfate, or supplements containing iron, calcium, or zinc can form insoluble complexes and significantly reduce quinolone absorption.30
Reduced fluorquinolone absorption due to interactions with multivalent cation drugs may be sufficient to result in ineffective clinical antibacterial effects.31,32
The interaction can be managed by timing with respect to when the fluorquinolone is given, either sufficiently before or after the cation-containing agent.30
Patients should be explicitly instructed not to take their antibiotic dose with milk, calcium-fortified juice, multivitamins, or antacids.33
Drugs affecting QT interval
See the section above here
Hypoglycemic agents
See the section above here
Cytochrome P450 inhibition
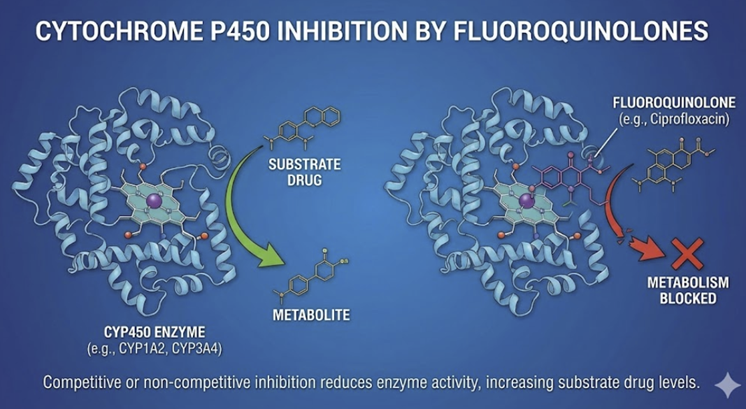 |
Some fluoroquinolones are inhibitors of hepatic CYP enzymes, notably CYP1A2.
Ciprofloxacin and enoxacin34 (Not available in the United States) administration may increase levels of drugs metabolized by the cytochrome p450 isoform,CYP1A2.
Theophylline clearance is reduced by ciprofloxacin, potentially causing theophylline toxicity (seizures, arrhythmias).35,36
Caffeine is also metabolized by CYP1A2; therefore, patients receiving ciprofloxacin may exhibit increase caffeine effects such as jittery notice or insomnia given that caffeine clearance is reduced due to inhibition of the CYP1A2 isoform.37
The muscle relaxant tizanidine is also metabolized by CYP1A2.
Tizanidine, an α-2 adrenergic agonist used in the treatment of muscle spasms secondary to multiple cirrhosis, spinal cord injury and other medical conditions, interaction with ciprofloxacin may be severe as the result of reduced blood pressure and sedation secondary to high tizanidine levels. Tizanidine in the presence of ciprofloxacin may exhibit a 5-20 times increase in plasma concentration.38
Tizanidine administration concurrent with ciprofloxacin is contraindicated.39
Warfarin metabolism may be partially inhibited by fluoroquinolones as well. Case reports describe elevated INR and bleeding risk when warfarin is combined with a quinolone. This interaction is unpredictable (not every patient), but close monitoring of INR is recommended and empiric warfarin dose reductions are sometimes made when starting a quinolone.40
July, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |