Medical Pharmacology Chapter 35 Antibacterial Drugs
Quinolones
Specific Quinolone Class Antibacterial Drugs
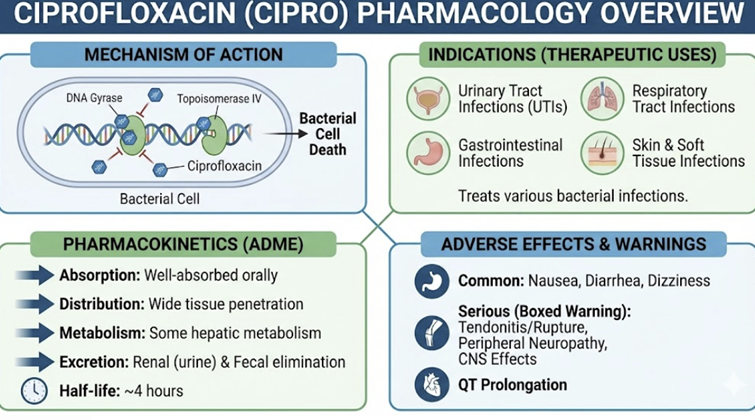
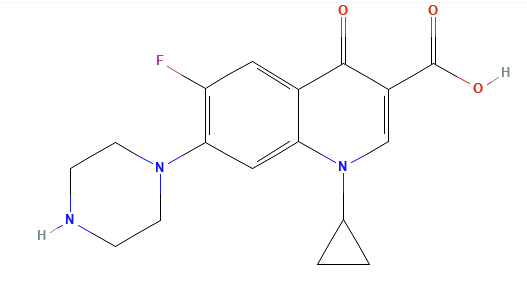
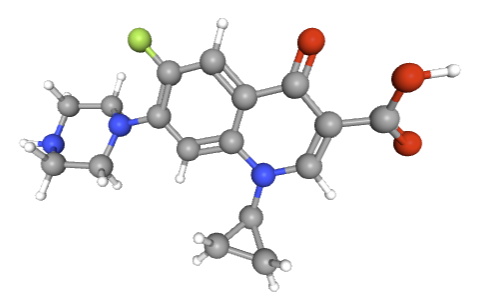
Ciprofloxacin (Cipro)1
Ciprofloxacin
Ciprofloxacin is especially potent against bacteria
belonging to the Enterobacteriaceae class such as
E. coli, Salmonella, Shigella, and
Klebsiella.
"Low-temperature electron micrograph
of the cluster of E. coli bacteria, magnify 10,000
times.
"Each individual bacterium is oblong
shaped. (March 2005)
Attribution
Photo by Eric Erbe, digital
colorization by Christopher Pooley, both of
USDA, ARS, EMU., Public domain, via Wikimedia
Commons
https://commons.wikimedia.org/wiki/File:E_coli_at_10000x,_original.jpg
"Color-enhanced scanning electron
micrograph showing Salmonella Typhimurium (red)
invading cultured human cells." (November 10, 2002
Attribution
Rocky Mountain Laboratories,
NIAID, NIH All the images, except specified ones
from the World Health Organization (WHO), are in
the public domain. For the public domain images,
there is no copyright, no permission required,
and no charge for their use.
Attribution
Center for Disease Control (CDC):
Shigella-Shigellosis
Attribution
Center for
Disease Control (CDC): Klebsiella
Ciprofloxacin
is likely the most active fluorquinolone against
Pseudomonas aeruginosa.
Gram-positive coverage is more limited with the drug
exhibiting only modest activity against Staphylococcus
aureus (MSSA) and Streptococcus species.
This resistance profile limits the use of
ciprofloxacin in pneumococcal pneumonia.
Ciprofloxacin therefore would not be first-line
empirical treatment for respiratory tract
infections if the primary pathogen is
penicillin-susceptible Streptococcus pneumoniae.
Ciprofloxacin is also useful in treating "atypical"
pathogens including Legionella, Mycoplasma,
and Chlamydia.
Ciprofloxacin is not considered effective in
treating infections due to anaerobes.1
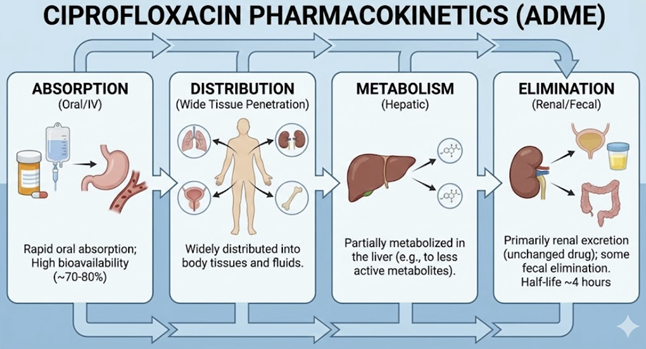
Ciprofloxacin: Pharmacokinetics Oral bioavailability ~70%-80%.2
Given the oral availability of about 75% and an IV dose with the
bioavailability of 100%, the oral dose would theoretically
require about 1.25 times the IV dose in order to obtain of
similar tissue exposure. Peak plasma levels (after a 250 mg oral dose) are around 1.25
µg/mL. Ciprofloxacin’s Vd is large (~2–3 L/kg total distribution),
indicating extensive tissue penetration.2
Ciprofloxacin exhibits 83 compartment distribution model with
the central compartment volume estimated at 0.161 L/kg.
The
range for the total volume of distribution is 2-3 L/kg.2,4
Protein binding is low (20–40%).4
Ciprofloxacin is metabolized in the liver (CYP1A2 substrate) to
four metabolites (each ~3–8% of dose), but the majority of the
drug is excreted unchanged: ~30% in urine (after oral dose) and
another fraction (~62%) in feces.2
Attribution
Metabolism Section
Ciprofloxacin. DrugBank.
https://go.drugbank.com/drugs/DB00537 The elimination half-life is about
4 hours.2
Ciprofloxacin achieves very high concentrations in the urinary
tract (making it ideal for UTIs)6 and also
penetrates well into prostate, lungs, bile, and bone.5
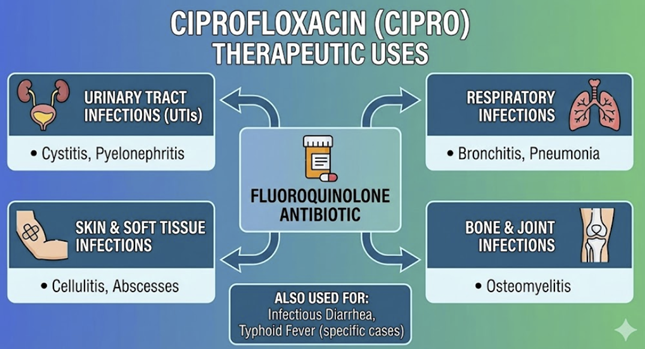
Ciprofloxacin Therapeutic Uses
Ciprofloxacin is first-line agent for
complicated UTIs
and an important option for
prostatitis
that may require 4–6 weeks treatment to clear chronic
bacterial prostatitis).1
Ciprofloxacin
is a drug of choice for
infectious diarrhea
(bacterial gastroenteritis) caused by organisms like
Traveler’s diarrhea
(enterotoxigenic E. coli)
Campylobacter jejuni, Shigella boydii, Shigella
dysenteriae or Shigella flexneri or Shigella
sonnei.
In
intra-abdominal infections, ciprofloxacin combined with
metronidazole for coverage of anaerobic Gram-negative bacilli is
used for diverticulitis9
or abdominal abscess.11
Historically abdominal abscess was often favored, in combination
with metronidizole for treating intra-abdominal infections.10
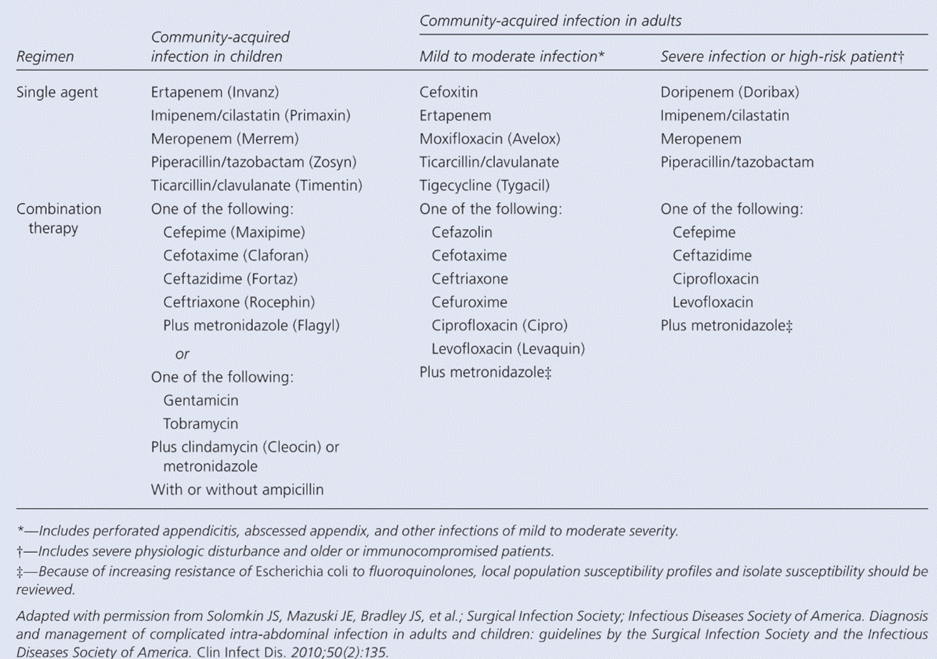
More recently, a number of agents have been identified that have
proveneffective in treating abdominal abscess. The choice of the
agent, either single agent treatment or combination therapy,
was classified on the basis of the infection being at community
acquired infection in children or community acquired infection
in adult with the infection being of varied severity. (2010)
Attribution
The table above is the slight
adaptation of table 2 of reference 11
Armstong
C Updated Guidelines on Diagnosis and Treatment
of Intra-abdominal Infections. Am Fam Physician.
2010;82(6): 694-709.
https://www.aafp.org/pubs/afp/issues/2010/0915/p694.html
However,
ciprofloxacin is not generally recommended as
first-line therapy for pneumonia due to limited efficacy against
such, pneumonia-causative bacteria e.g. Streptococcus
pneumoniae.
Ciprofloxacin does have a role in particular situations in
which pneumonia is caused by Pseudomonas aeruginosa or
certain Gram-negative bacteria in the hospital-acquired
pneumonia setting.15
Ciprofloxacin
is indicated for bioterrorism-related infections: it is the first-line
prophylaxis and treatment for
inhalational anthrax
(post-exposure to B.
anthracis)16
Ciprofloxacin
is also recommended for
plague (Yersinia
pestis) treatment.
Ciprofloxacin is generally well-tolerated but has typical
fluoroquinolone precautions (see next section below).
A
notable distinction is that ciprofloxacin is one of the
fluoroquinolones that
inhibit CYP1A2,
which can raise levels of drugs like
theophylline,
caffeine,
and tizanidine.18
Concurrent administration of theophylline use can lead to
toxicity (seizures, arrhythmias) due to this interaction, so
dose adjustment and monitoring may be required.19
Ciprofloxacin also may increase warfarin levels/effects, so INR
(Prothrombin time) monitoring is needed when co-administered.20
Like all systemic quinolones, it carries a risk of
tendonitis/tendon rupture, neuropathy, CNS effects, etc.,
especially in older patients.21 Because of limited pneumococcal activity, it should
not be used
for community-acquired respiratory infections where
S. pneumoniae is
suspected.
![]() Ciprofloxacin
is a prototypical fluoroquinolone with very good activity
against Gram-negative bacterial organisms.1
Ciprofloxacin
is a prototypical fluoroquinolone with very good activity
against Gram-negative bacterial organisms.1
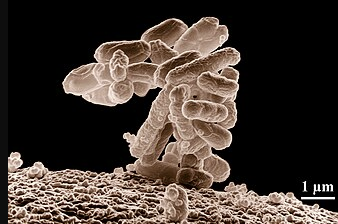
E. coli
Salmonella Typhimurium
Shigella
Klebsiella
![]() However, Streptococcus pneumoniae is often
either resistant or only intermediate insensitivity.
However, Streptococcus pneumoniae is often
either resistant or only intermediate insensitivity.

![]() Ciprofloxacin
is widely used for urinary tract infections, including
pyelonephritis
and prostatitis,
due to its strong activity against uropathogens and high urinary
level.1,7,8
Ciprofloxacin
is widely used for urinary tract infections, including
pyelonephritis
and prostatitis,
due to its strong activity against uropathogens and high urinary
level.1,7,8
![]() Even more
recently, a very complete, evidenced-based set of guidelines for
managing intra-abdominal infection has been published, including
the role of ciprofloxacin as well as the number of other agents
depending on the specific circumstances.12
Even more
recently, a very complete, evidenced-based set of guidelines for
managing intra-abdominal infection has been published, including
the role of ciprofloxacin as well as the number of other agents
depending on the specific circumstances.12 ![]() Ciprofloxacin’s
excellent Gram-negative coverage makes it useful in
hospital-acquired
infections13 for example for
febrile
neutropenia
in oncology patients it can be part of prophylaxis or therapy14.
Ciprofloxacin’s
excellent Gram-negative coverage makes it useful in
hospital-acquired
infections13 for example for
febrile
neutropenia
in oncology patients it can be part of prophylaxis or therapy14.
![]() Safety Concerns
Safety Concerns
![]() Levofloxacin (3rd Generation)
Levofloxacin (3rd Generation)
 |
 |
Spectrum:
Levofloxacin, the L-isomer of ofloxacin exhibiting about twice the potency, is often termed a “respiratory fluoroquinolone.” Respiratory quinolones, as monotherapy, exhibits shows an advantage in clinical cure compared to a beta-lactam combined with a macrolide antibiotic.23
Levofloxacin and moxifloxacin are examples of respiratory quinolones.23
Levofloxacin has broad activity against Gram-positive Streptococcus pneumoniae (including many penicillin-resistant strains) and Staph aureus (MSSA), while retaining excellent Gram-negative coverage (most Enterobacteriaceae, H. influenzae, M. catarrhalis, etc.)24
Its Pseudomonas activity is moderate, not as potent as ciprofloxacin, but high-dose levofloxacin can treat Pseudomonas in urinary or respiratory infections if susceptible.24
The FDA has suggested that the ofloxacin be used only for strongly suspected bacterial infections in order to reduce the likelihood of drug-resistance bacteria development. Furthermore, levofloxacin is not recommended for empiric use in individuals at the risk of multi-drug-resistant Escherichia coli. Levofloxacin is recommended for infections due to extended-spectrum betalactamase producing enterobacterales, AmpC-E beta-lactamase-produces enterobacterales or Stenotrophomonas maltophilia (mild).
AmpC are clinically notable cephalosporinases encoded in many of the Enterbacteriaceae and some other bacteria, mediating resistance to many antibiotics including most penicillins and ß-lactamase inhibitor-ß-lactam combinations.25
Levofloxacin is also active against atypicals (Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumoniae) making it effective for atypical pneumonia.26
Levofloxacin exhibits limited activity against anaerobes; therefore, levofloxacin is not a first-line anaerobic drug.27
Pharmacokinetics28
Levofloxacin’s pharmacokinetic profile is favorable with nearly 100% oral bioavailability.
Oral and IV doses are interchangeable (500 mg IV = 500 mg PO yields equivalent exposure). Absorption is rapid (Tmax ~1–2 h).
Distribution is wide (Vd ~1.1–1.3 L/kg) penetrating well into lung tissue, bronchial secretions, sinuses, skin, and urinary tract.
Protein binding is ~30% (24-38%)
Levofloxacin undergoes minimal metabolism (<5%)
 |
The vast majority is excreted unchanged in urine with about 85–87% of a dose recovered unchanged in the urine within 48 hours.
Its elimination half-life is approximately 6-8 hours.
If the patient presents with renal impairment, dose or interval may require adjustment.24
Therapeutic uses:
Levofloxacin is often used to treat respiratory tract infections.
Levofloxacin, frequently used for monotherapy to cover both typical and atypical pneumonia pathogens, is often administered in treating community-acquired pneumonia (CAP).29
![]() Guidelines consider
respiratory fluoroquinolones (levofloxacin or moxifloxacin) as
first-line options for CAP in patients with comorbidities or
requiring hospitalization.23
Guidelines consider
respiratory fluoroquinolones (levofloxacin or moxifloxacin) as
first-line options for CAP in patients with comorbidities or
requiring hospitalization.23
![]() Levofloxacin
may be used for treating
acute bacterial
sinusitis and
acute exacerbations
of chronic bronchitis; however, the FDA has advised that
fluoroquinolones should be used only in patients who
have no other treatment options for managing acute
bacterial sinusitis, acute bacterial exacerbation of
chronic bronchitis and uncomplicated urinary tract
infections. This update from the FDA reflects the
conclusion that side effect risk usually outweighs
the benefit for these patients. The exception would
be in certain serious bacterial infections in which
case the benefits of fluroquinolone administration
outweigh the risks.30
Levofloxacin
may be used for treating
acute bacterial
sinusitis and
acute exacerbations
of chronic bronchitis; however, the FDA has advised that
fluoroquinolones should be used only in patients who
have no other treatment options for managing acute
bacterial sinusitis, acute bacterial exacerbation of
chronic bronchitis and uncomplicated urinary tract
infections. This update from the FDA reflects the
conclusion that side effect risk usually outweighs
the benefit for these patients. The exception would
be in certain serious bacterial infections in which
case the benefits of fluroquinolone administration
outweigh the risks.30
Beyond respiratory uses, levofloxacin is used for complicated urinary tract infections,30
A complicated urinary tract infection is an infection associated with an elevated treatment failure risk.
For example, a complicated UTI diagnosis would be appropriate in immunocompromised patients males, pregnant patients and those patients presenting with fever, stones, sepsis, catheters, urinary obstruction or kidney involvement.31
Levofloxacin may be appropriate in treating pyelonephritis.32
Although fluoroquinolones and trimethoprim/sulfamethoxazole in most cases will be effective oral antibiotics for treating pyelonephritis, increasing resistance make empiric use more problematic.
Hospitalized patients would more likely receive parenteral antibiotic treatment and those with sepsis or infection risk with the multidrug-resistant bacteria should probably receive antibiotics exhibiting activity against extended-spectrum beta-lactamase-producing organisms.32
Levofloxacin appears to be a reasonable choice for treating prostatitis.can be used for prostatitis.33
Levofloxacin, described as the second-line treatment) is an alternative agent for tuberculosis.34
Skin and soft tissue infections Is an FDA approved indication for levofloxacin use.24
Levofloxacin (like ciprofloxacin) is FDA-approved for inhalational anthrax post-exposure35 and for plague treatment/prophylaxsis.36
Tolerability and special points:
Levofloxacin does not inhibit cytochrome P450 significantly, so it has fewer drug interactions than ciprofloxacin.37
Levofloxacindoes not prolong the QT interval to a significant degree at least compared to most other fluoroquinolones. Moxifloxacin has more pronounced effect.
When analyzing several fluoroquinolones with respect to the risk of inducing ventricular arrhythmias in Korea's general population, ciprofloxacin and levofloxacin, during the week after administration, were not associated with increased risk; whereas ofloxacin showed reduce risk and moxifloxacin increase the risk of serious arrhythmias.38
The results of the study may suggest that levofloxacincan could be a safer choice in patients at risk for arrhythmias.
Nonetheless, caution is still advised with other QT-prolonging drugs.
![]() Like all fluoroquinolones, levofloxacin can cause tendinopathy, CNS
effects (insomnia, dizziness), and rarely peripheral neuropathy
or dysglycemia.
Like all fluoroquinolones, levofloxacin can cause tendinopathy, CNS
effects (insomnia, dizziness), and rarely peripheral neuropathy
or dysglycemia.
Because of the risk of side effects, in 2016 the FDA recommended that for uncomplicated sinusitis, bronchitis, and uncomplicated UTIs, fluoroquinolones (including levofloxacin) should be reserved for patients who have no alternative treatment options.
In practice, this recommendation cautions that primary care clinicians use levofloxacin for these common infections only if first-line antibiotics (e.g. amoxicillin-clavulanate for sinusitis, or trimethoprim-sulfamethoxazole for UTI) cannot be used.30
Levofloxacin and fluoroquinolones in general have in the past been contraindicated in children and pregnant/nursing women due to the class’s cartilage toxicity concerns; however, this use been challenged since the evidence is weak and the American Academy of pediatrics recommend fluoroquinolones as second-line antibiotic with restricted uses.5
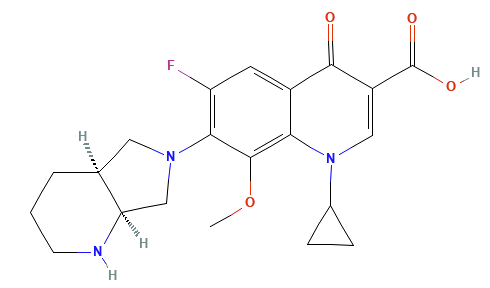
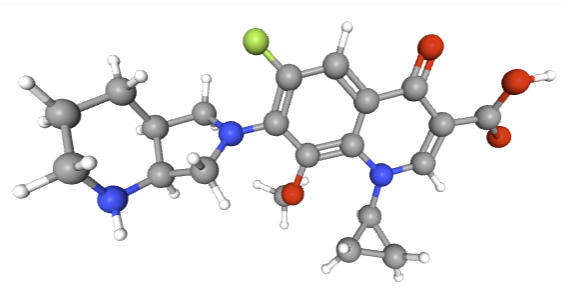
Moxifloxacin (3rd/4th Generation
)
 |
 |
Spectrum
Moxifloxacin is a “fourth-generation” fluoroquinolone with broad coverage including Gram-positives, atypicals, and some anaerobes.39,40
Moxifloxacin exhibits excellent activity against Streptococcus pneumoniae (including PRSP), Streptococcus pyogenes, and good activity against Methicillin-Susceptible Staphylococcus Aureus (MSSA) (but not Methicillin-Resistant Staphylococcus Aureus, MRSA).
Gram-negative spectrum of moxifloxacin:
Haemophilus, Moraxella
(Many) Enterobacteriaceae, but not Pseudomonas
Moxifloxacin is notable in its activity against anaerobic bacteria such as Bacteroides fragilis.42
Because of this activity, moxifloxacin can be used in some polymicrobial intra-abdominal infections as a single agent.41
Moxifloxacin is also effective in covering atypical bacterial infections due to for example Mycoplasma, Chlamydia, Legionella.43
Pharmacokinetics44
Moxifloxacin is very well absorbed orally (~85–90% bioavailability) allowing once-daily oral dosing of 400 mg to achieve therapeutic levels similar to IV.
Moxifloxacin is widely distributed, penetrating lung tissue, sinuses, etc., (Vd ~1.7–2.7 L/kg).
Moxifloxacin’s clearance is predominantly hepatic via conjugation (glucuronidation and sulfation).
This drug is not metabolized utilizing the cytochrome p450 drug metabolizing system; therefore, drug-drug interactions depending on cytochrome p450 are not of concern.
About half an administered dose is excreted as unchanged drug: ~20% in urine and ~25% in feces with the remainder excreted as metabolites.
Because only 20% of the drug Is excreted in urine, moxifloxacin achieves relatively low urinary concentrations and is therefore not typically appropriate for treating urinary tract infections.
The relatively long half-life, ~12–15 hours, provides the rationale for once-daily dosing.
Since the kidneys do not represent the major drug elimination pathway, a moxifloxacin dose adjustment is Not required in the presence of renal insufficiency. By contrast, in severe hepatic impairment moxifloxacin should be used cautiously or avoided due to reduced metabolism.
Therapeutic uses39,45,46,47
![]() Moxifloxacin is primarily used for
respiratory
infections. It is indicated for
community-acquired pneumonia, including aspiration
pneumonia (due to its anaerobe coverage) and is very
effective for community acquired pneumonia (CAP), including cases with high
penicillin-resistant pneumococci.
Moxifloxacin is primarily used for
respiratory
infections. It is indicated for
community-acquired pneumonia, including aspiration
pneumonia (due to its anaerobe coverage) and is very
effective for community acquired pneumonia (CAP), including cases with high
penicillin-resistant pneumococci.
Moxifloxacin has been FDA-approved for treating CAP infections due to susceptible Streptococcus pneumonia strains and Mycoplasma pneumonia strains.
This agent is also approved for treating:
Acute bacterial exacerbation of chronic bronchitis48
Acute bacterial sinusitis
Complicated skin and skin structure infections, including abscessus, cellulitis and surgical wound infections.
![]() Other
uses include uncomplicated skin and skin structure
infections, complicated inter-abdominal infections as well
as treatment of plague (pneumonic and septicemic forms) as
result of Yersinia pestis infections.39
Other
uses include uncomplicated skin and skin structure
infections, complicated inter-abdominal infections as well
as treatment of plague (pneumonic and septicemic forms) as
result of Yersinia pestis infections.39
Moxifloxacin monotherapy is effective in treating polymicrobial intra-abdominal infections of mild-to-moderate severity.49
Moxifloxacin is one of the options for bacterial conjunctivitis as a topical ophthalmic solution, owing to its broad coverage.50
Safety considerations
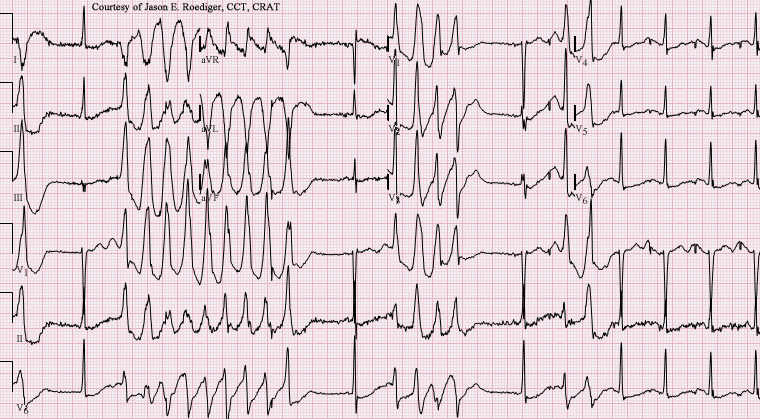
QT interval prolongation is an adverse effect of moxifloxacin administration51 and appears present to a greater degree relatives other fluroquinolone antibiotics.
Moxifloxacin can cause QT prolongation and even torsades de pointes in rare cases.52
 |
|
 |
|
Therefore, it is contraindicated in patients with known QT prolongation or receiving antiarrhythmic drug therapy,39 and caution is urged if other QT-prolonging risk factors are identified.
Unlike levofloxacin, moxifloxacin should be avoided in patients with significant arrhythmia risk factors.
Other adverse effect profiles are similar to fluroquinolone effects (GI upset, CNS effects, etc.).
![]() Moxifloxacin
in pregnancy should be evaluated on a case-by-case basis,
which considers balancing benefits and risk to the
developing fetus.39
Moxifloxacin
in pregnancy should be evaluated on a case-by-case basis,
which considers balancing benefits and risk to the
developing fetus.39
The safety of moxifloxacin has not been established for use in patients under the age of 18.53
![]() "Moxifloxacin may cause problems with bones, joints, and
tissues around joints in children. Moxifloxacin should not
be given to children younger than 18 years old."54
"Moxifloxacin may cause problems with bones, joints, and
tissues around joints in children. Moxifloxacin should not
be given to children younger than 18 years old."54
Other Quinolones
Ofloxacin:
A second-generation agent, ofloxacin is the racemic mixture from which levofloxacin (the L-isomer) is derived.
Ofloxacin has broad activity similar to levofloxacin but slightly less potency against susceptible strains of Streptococcus pneumoniae given that half the mixture is the less-active D-isomer.55
Susceptible bacteria include:55
Aerobic Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyrogenes
Aerobic Gram-negative bacteria
Citrobacter koseri (Citrobacter diversus)
Enterobacter aerogenes
Escherichia coli
Haemophilus influenzae
Neisseria gonorrhoeae
![]() Fluoroquinolones,
including ofloxacin, used to be favored for gonorrhea treatment
(1980s). Fluoroquinolones, due to resistance development by
Neisseria gonorrhoeae, were generally no longer used by the late
1990s.
Fluoroquinolones,
including ofloxacin, used to be favored for gonorrhea treatment
(1980s). Fluoroquinolones, due to resistance development by
Neisseria gonorrhoeae, were generally no longer used by the late
1990s.
Proteus mirabilis
Pseudomonas aeruginosa
Other bacteria:55
Chlamydia trichomonas
Ofloxacin may be used to treat is used for UTIs, prostatitis, certain infectious diarrheas.aOfloxacin’s half-life ~7–8 hours, and it is dosed BID.
Gemifloxacin
 |
 |
|
A newer respiratory fluoroquinolone (oral only) indicated for mild-to-moderate community-acquired pneumonia and acute exacerbation of bronchitis.56
Gemifloxacin may be used to treat bacterial infections caused by susceptible strains including:
Streptococcus pneumoniae (S. pneumoniae)
Haemophilus influenzae (H. influenzae)
Moraxella catarrhalis (M catarrhalis)
Multidrug-Resistant Streptococcus pneumoniae (MDRSP)
Chlamydia pneumoniae (C. pneumoniae)
Klebsiella pneumoniae (K. Pneumoniae)
![]() Adverse Effect:57
Adverse Effect:57
In some patients, typically female, an uncommon delayed onset skin rash may develop. The presence of asthma tend to increase the risk of the skin rash.57
The combination of this side effect in the narrow antibacterial indications for gemifloxacin may have limited its clinical use.
The overall adverse effect profile for gemifloxacin is in accord with that of the fluoroquinolone class.58
Delafloxacin59
This fluoroquinolone is
active against MRSA
in addition to Pseudomonas and other typical organisms.
Delafloxacin is
indicated for acute
bacterial skin and skin structure infections and also
approved for community-acquired bacterial pneumonia.
Delafloxacin may be used to treat I variety of bacterial
infections caused by susceptible strains such as:
Gram-positive organisms:
Staphylococcus aureus (including methicillin-resistant [MRSA]
and methicillin-susceptible [MSSA] isolates.
Staphylococcus haemolyticus
Staphylococcus lugdunensis
Staphylococcus agalactiae
Staphylococcus anginosus group [Staphylococcus
anginosus, Streptococcus intermedius, and
Streptococcus constellatus]
Streptococcus pyrogenes
Enterococcus faecalis
Gram-negative organisms:
Escherichia coli
Enterobacter cloacae
Klebsiella pneumoniae
Pseudomonas aeruginosa
Susceptible bacteria for Community-Acquired Bacterial
Pneumonia (CABP)
Streptococcus pneumoniae
Staphylococcus aureus (methicillin-susceptible MSSA
isolates only
Klebsiella pneumoniae
Escherichia coli
Pseudomonas aeruginosa
Haemophilus influenzae
Haemophilus parainfluenza
Chlamydia pneumoniae
Legionella pneumophila
Mycoplasma pneumoniae
Delafloxacin does not appear to cause QT
prolongation to a significant degree. However, Delafloxacin’s
side effect profile still includes tendon and neuropathy
warnings.60
July, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |