Medical Pharmacology Chapter 35 Antibacterial Drugs
Sulfonamides
Sulfonamides: Comparative Pharmacokinetics
|
Drug |
Route of Administration |
Oral Bioavailability |
Primary Site of Action |
Protein Binding |
Key Metabolic pathways |
Elimination Half-life |
Primary Excretion Route |
Note |
|
Sulfamethoxazole |
Oral |
85%-90%1 |
Systemic |
About 70%1 |
Hepatic:N-acetylation, Oxidation (CYP2C9)1 |
About 10 hours1 |
Renal2 |
Systemic component of TMP/SMX for wide range of infections |
|
Sulfasalazine |
Oral |
Less than 15% parent drug; about 60% as metabolite3 |
Colon |
>99% (parent drug); about 70% as sulfapyridine metabolite3 |
Intestinal cleavage of active components; hepatic acetylation of sulfapyridine3 |
About 7.6 hours for parent drug; 10-15 hours for metabolite3 |
Fecal (5-ASA); renal (sulfapyridine)3 |
Prodrug: targeted anti-inflammatory colon action for IBD |
|
Sulfadiazine |
Oral, topical, as silver sulfadiazine |
Well absorbed4 |
Systemic |
38-48%5 |
Hepatic acetylation5 |
7-17 hours5 |
Renal5 |
Elevated risk of crystalluria because of low drug and metabolite urinary solubility |
Table References
(1) Sulfamethoxazole. DrugBank. https://go.drugbank.com/drugs/DB01015
(2) Bactrim: sulfamethoxazole and trimethoprim FDA drug label: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/017377s084lbl.pdf
(3) Azulfidine Clinical Pharmacology (sulfasalazine). Pfizer Medical. https://www.pfizermedical.com/azulfidine/clinical-pharmacology
(4) Sulfadiazine https://www.glowm.com/resources/glowm/cd/pages/drugs/s030.html
(5) Sulfadiazine. https://en.wikipedia.org/wiki/Sulfadiazine
Profiles of Some Key Sulfonamides
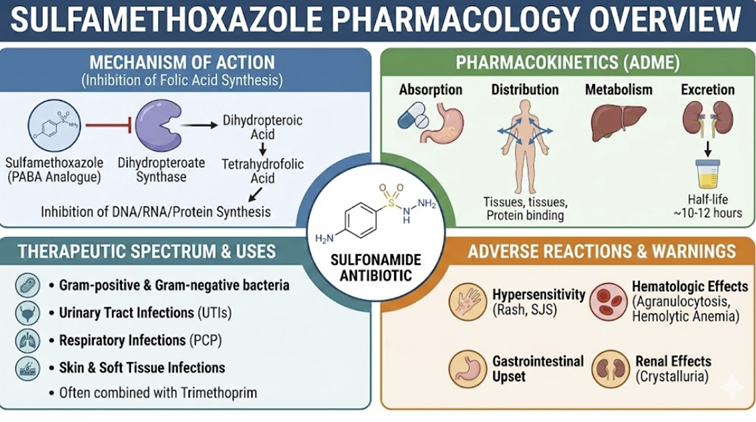
Sulfamethoxazole (SMX) is particularly important given that it is used in the important combination product (TMP/SMX).
 |
Absorption
It is rapidly absorbed orally with a high bioavailability of 85-90%. Peak plasma concentrations (Tmax) are reached within 1 to 4 hours.7
Distribution
SMX has a volume of distribution of approximately 13 L.
Sulfamethoxazole distributes into various tissues and fluids,
including sputum, vaginal fluid, and middle ear fluid. and is
approximately 70% bound to plasma proteins.
SMX is extensively metabolized in the liver.7,8
The primary pathway is N4-acetylation, mediated by N-acetyltransferase (NAT) enzymes.
A clinically significant secondary pathway is oxidation by the cytochrome P450 enzyme CYP2C9.
This oxidation can form a reactive hydroxylamine metabolite,
which is thought to play a role in idiosyncratic
hypersensitivity reactions.
Sulfamethoxazole is excreted renally, with about 30% as the free, active drug and the remainder as the inactive N4-acetylated metabolite.
Dose adjustments are necessary for patients with significant
renal impairment.
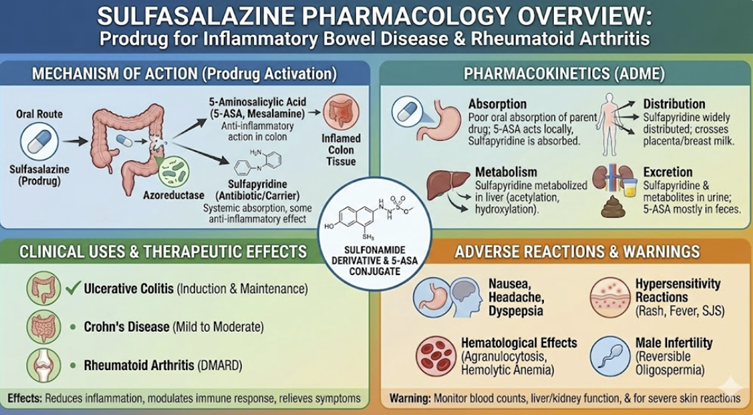 |
Less than 15% of an oral dose is absorbed as the intact parent drug in the small intestine.
The majority of the dose transits to the colon, where intestinal bacteria cleave its azo bond, releasing the two active components: sulfapyridine (SP) and 5-aminosalicylic acid (5-ASA).
Distribution & Action11,12
This differential absorption and metabolism is the key to its therapeutic effect.
The sulfapyridine moiety is well-absorbed from the colon (bioavailability ~60%) and is thought to be responsible for the systemic anti-inflammatory and immunomodulatory effects in conditions like rheumatoid arthritis.
In
contrast, the 5-ASA moiety is poorly absorbed (~10-30%) and
exerts its anti-inflammatory effects locally on the colonic
mucosa, making it effective for ulcerative colitis.
Absorbed sulfapyridine (SP) is metabolized in the liver, primarily via acetylation.
The rate of this metabolism is dependent on the patient's genetically determined NAT2 acetylator phenotype (i.e., "fast" or "slow" acetylators), which influences the half-life of SP.
The unabsorbed 5-ASA and its metabolites are largely excreted in the feces.11,12
Sulfadiazine (Oral and Tropical)
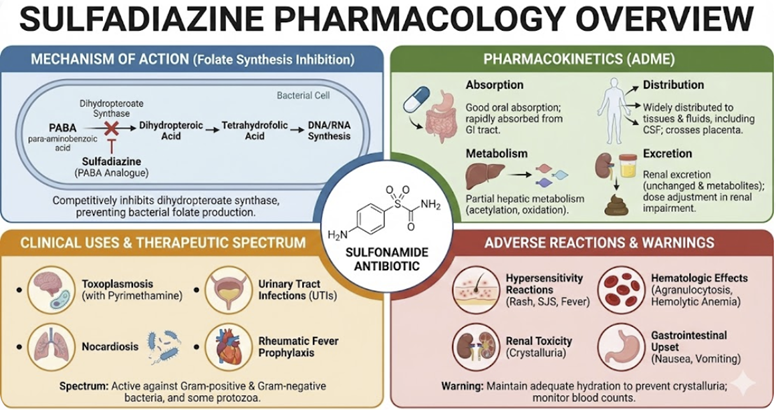 |
Absorption
Sulfadiazine is readily absorbed from the GI tract when given
orally.
When used topically as silver sulfadiazine cream on large burn areas, some systemic absorption of sulfadiazine can occur.14
Distribution
It is widely distributed in the body and is 38-48% bound to plasma proteins.
Metabolism & Excretion
It
is partially metabolized in the liver via acetylation and has an
elimination half-life of 7 to 17 hours.13
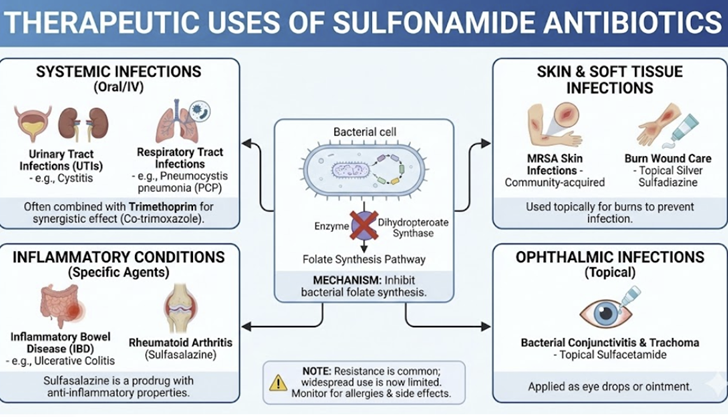
Therapeutics in Clinical Medicine
 |
|
![]() A
combination of trimethoprim sulfamethoxazole is an appropriate
and effective choice to treat a variety of infections such as:
Pneumocystis jiroveci pneumonia, prostatitis, urinary
tract infections as well as certain infections due to
susceptible strains of Shigella, Salmonella and
nontuberculosis mycobacteria.
A
combination of trimethoprim sulfamethoxazole is an appropriate
and effective choice to treat a variety of infections such as:
Pneumocystis jiroveci pneumonia, prostatitis, urinary
tract infections as well as certain infections due to
susceptible strains of Shigella, Salmonella and
nontuberculosis mycobacteria.
This combination (TMP-SMZ) retains activity against most Staphylococcus aureus strains (methicillin-susceptible and-resistant) as well as against respiratory tract bacteria such as Haemophilus species, Moraxella catarrhalis and Klebsiella pneumoniae.24
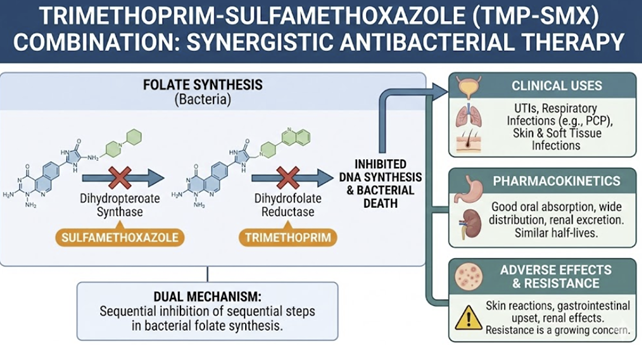 |
Urinary Tract Infections (UTIs):
TMP-SMX is an effective and important oral treatment for uncomplicated UTIs (cystitis) and an option for pyelonephritis, provided local E. coli resistance rates are not too high.
Strains of the following bacteria are responsible for urinary tract infections and include: Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis, and Proteus vulgaris.
For initial, uncomplicated urinary tract infections, a single, effective agent is preferred compared to combinations (Bactrim a.k.a. trimethoprim/sulfamethoxazole in this case is considered a single agent).8
Guidelines recommend a 3-day course of TMP-SMX for acute uncomplicated cystitis in women when the local resistance prevalence of uropathogens to TMP-SMX is < ~20%.
![]() For
treating urinary tract infections, trimethoprim/sulfamethoxazole
(TMP-SMX) is highly effective for susceptible bacteria.
For
treating urinary tract infections, trimethoprim/sulfamethoxazole
(TMP-SMX) is highly effective for susceptible bacteria.
Increasing resistance among E. coli, however, presents treatment challenges.
Prescribing based on empiric (experience) for UTIs probably may not be appropriate if local resistance among E. coli is greater than 20%.4 The same guidance would apply if the patient has recently received TMP-SMX.3
In this case, alternative first-line agents (like nitrofurantoin or fosfomycin) are preferred.4,6
Due to increasing E. coli resistance (often via sul and dfr genes), clinicians should use local antibiograms to guide empiric therapy.5
TMP-SMX is also effective in prophylaxis of recurrent UTIs:
Respiratory tract Infections
Sulfonamides are not first-line for common community-acquired pneumonia or sinusitis due to resistance of S. pneumoniae and H. influenzae in many regions.16
However, TMP-SMX can be used in acute exacerbations of chronic bronchitis in COPD (where H. influenzae or Moraxella may be targets). 17
Sulfonamides is also an alternative (though not preferred) for acute otitis media in children or sinusitis, particularly if the patient cannot take beta-lactams, as long as regional pneumococcal resistance is low.18
![]() Pneumocystis
pneumonia (PCP) in immunocompromised patients
(HIV/AIDS, transplant, etc.) is
optimally
treated with high-dose TMP-SMX.
Pneumocystis
pneumonia (PCP) in immunocompromised patients
(HIV/AIDS, transplant, etc.) is
optimally
treated with high-dose TMP-SMX.
It is the first-line treatment and prophylaxis for Pneumocystis jirovecii pneumonia (PjP) as no other drug has proven more effective.19
Trimethoprim/sulfamethoxazole is the drug of choice for both the treatment and prophylaxis of PjP.8,9,19
This opportunistic fungal infection is a major cause of morbidity and mortality in immunocompromised individuals, particularly those with HIV/AIDS, organ transplant recipients, and patients on chronic high-dose corticosteroids.
For this indication, TMP/SMX is
life-saving.
Dosing for PCP is higher and intravenous therapy is used for moderate-severe cases. 20
TMP-SMX for PCP prophylaxis is standard in AIDS patients with CD4 count <200, in transplant patients, etc.21
Sulfamethoxazole exhibits good penetration into lung tissue.22
Otolaryngologic infections
TMP-SMX is an option for chronic sinusitis or otitis media when first-line agents fail or cannot be used.23
It is ineffective for streptococcal pharyngitis as it does not eradicate Group A strep and thus cannot prevent rheumatic fever.19
For acute otitis media in children and for acute maxillary sinusitis in adults (as the result of infection by susceptible Haemophilus influenzae and Streptococcus pneumoniae strains) trimethoprim-sulfamethoxazole would be a reasonable, effective treatment.3
Skin and Soft Tissue Infections
Oral TMP-SMX has emerged as an important drug for community-acquired MRSA (CA-MRSA, Community-Associated Methicillin-Resistant Staphylococcus Aureus) skin and soft tissue infections, such as:
Cellulitis
Abscesses, and
Wound infections.25,9
CA-MRSA isolates are often susceptible to TMP-SMX (even if resistant to beta-lactams), making it a recommended oral agent for outpatient therapy of uncomplicated MRSA infections.19
The IDSA MRSA guidelines list TMP-SMX as a first-line option for purulent skin infections caused by MRSA, in addition to clindamycin or doxycycline.26
Dosing is typically 1-2 DS tablets BID for 7–10 days, depending on severity.27
Gastrointestinal Infections
TMP-SMX can be used for some bacterial diarrheal illnesses.
It is effective against traveler’s diarrhea caused by enterotoxigenic E. coli and is an option (though fluoroquinolones are more commonly used, resistance patterns permitting)28,29
TMP-SMX is effective for treating shigellosis (caused by Shigella) but only in regions where the organism is still susceptible.
There is widespread resistance to trimethoprim-sulfamethoxazole as well as many other antibiotics. So other agents including ß-lactams, quinolones, azithromycin are more likely to be effective.30
For cholera (Vibrio cholerae), TMP-SMX is an alternative to tetracyclines.31
In cyclosporiasis and isosporiasis (protozoal diarrheal infections), TMP-SMX is the recommended treatment and prophylaxis.32,33
Nocardiosis
Infection with Nocardia (a Gram-positive branching filamentous bacterium) is traditionally treated with sulfonamides.34
TMP-SMX is first-line therapy for Nocardia infections (e.g. Nocardia pneumonia or brain abscess), given its effectiveness against most Nocardia species.34,35
Relatively slow response requires higher than usual doses for extended time (many months).35
 |
|
Parasitic and Fungal Infections
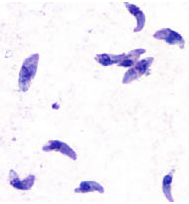
Toxoplasmosis
Toxoplasmosis of
the brain (toxoplasmic encephalitis) in immunosuppressed
patients is treated with sulfadiazine + pyrimethamine plus
leucovorin rescue.36 If a patient with toxoplasmosis has a sulfonamide allergy,
clindamycin + pyrimethamine is used as an alternative, or
high-dose TMP-SMX has also shown efficacy.36
Attribution:
DPDx Image Library, Public
domain, via Wikimedia Commons
https://commons.wikimedia.org/wiki/File:Toxoplasma_gondii_tachy.jpg
Pneumocystis
jirovecii pneumonia: TMP-SMX is first-line for both treatment and prophylaxis.19
Malaria:
sulfadoxine-pyrimethamine (Fansidar) was a key therapy for
Plasmodium
falciparum (especially chloroquine-resistant strains)
and is still used intermittently for prevention in pregnant
women in Africa. 37,38
Chloroquine is the preferred malaria treatment providing the
parasite is sensitive to the drug.39
Resistance to chloroquine is widespread and typically
alternative medications must be used.
Examples of alternative drugs include the combination of
artemether-lumefantrine and artesunate-mefloquine.39
Brucellosis Brucellosis, a bacterial zoonosis, can be treated with a
combination that includes TMP-SMX (e.g. TMP-SMX + rifampin)
as a second-line regimen.40
Doxycycline, gentamicin, streptomycin and rifampin may also
be used.40
Isosporiasis
and cyclosporiasis
This infection due to intestinal coccidian parasites are
effectively treated with TMP-SMX.32,33
Burn wound prophylaxis and other topical uses
Silver
sulfadiazine cream is widely used in burn units and
emergency settings to prevent burn wound infections.41
Effectiveness against many Gram-positive and Gram-negative
bacteria (and some Candida) is due to released silver ions.
Burn patients often receive daily topical silver
sulfadiazine as standard care, with reapplication if needed
to prevent wound sepsis.41
July, 2025
Werth B Trimethoprim M
Sulfamethoxazole. Merck manual professional version.
(Reviewed/revised May 2024.
https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-medications/trimethoprim-and-sulfamethoxazole
Langner J Treatment options for
urinary tract infections | Understanding UTIs, Part
5. Stanford Medicine: June 11, 2020.
https://med.stanford.edu/news/insights/2020/06/treatment-options-for-urinary-tract-infections-understanding-utis-part-5.html
MacDougal DNA Disruptors:
Sulfonamides, Quinolones, and Nitroimidazoles.
Chapter 57. In Goodman & Gilman's The
Pharmacological Basis of Therapeutics (Brunton LL
Knollman BC eds) McGraw Hill LLC (2023).
Jancel T Dudas V Management of
uncomplicated urinary tract infections. West J Med.
2002 January;176(1): 51-55.
https://pmc.ncbi.nlm.nih.gov/articles/PMC1071654/
Smit C Keighley C Rogers K Miyakis
S Taxis K Robertson H Pont L Temporal Trends of
Escherichia coli Antimicrobial Resistance and
Antibiotic Utilization in Australian Long-Term Care
Facilities. Antibiotics 2025 14(2), 208.
https://www.mdpi.com/2079-6382/14/2/208
Bono M Leslie S Uncomplicated
Urinary Tract Infections. StatPearls. National
Library of Medicine Bookshelf. (Last update:
February 21, 2025).
https://www.ncbi.nlm.nih.gov/books/NBK470195/
Sulfamethoxazole. DrugBank.
https://go.drugbank.com/drugs/DB01015
Bactrim (sulfamethoxazole and
trimethoprim). FDA.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/017377s084lbl.pdf
Kemnic T Coleman M Trimethoprim
Sulfamethoxazole. StatPearls. National Library of
Medicine Bookshelf. (Last update: November 28,
2022).
https://www.ncbi.nlm.nih.gov/books/NBK513232/
Sulfasalazine. DrugBank.
https://go.drugbank.com/drugs/DB00795
Azulfidine Clinical Pharmacology (sulfasalazine). Pfizer
Medical.
https://www.pfizermedical.com/azulfidine/clinical-pharmacology
Choi J Patel P Fenando
Sulfasalazine. StatPearls. National Library of
Medicine Bookshelf. (Last update: March 21, 2024).
https://www.ncbi.nlm.nih.gov/books/NBK557809/
Sulfadiazine.
https://en.wikipedia.org/wiki/Sulfadiazine
Silver sulfasalazine. DrugBank.
https://go.drugbank.com/drugs/DB05245
Jung W Koo H Kim K Shin S Kim S
Park Y Antibacterial Activity and Mechanism of
Action of the Silver Ion in Staphylococcus aureus
and Escherichia coli. Appl Environ Microbio. 2008
February 1;74(7): 2171-2178.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2292600/
Iyer U Pneumococcal Infections
(Streptococcus pneumoniae) Medication. (Updated:
February 6, 2025). Medscape.
https://emedicine.medscape.com/article/225811-medication
Nouira S Marghli S Besbes L Boukep
R Daami M Nciri N Elatrous S Aboung F Standard
versus Newer Antibacterial Agents in the Treatment
of Severe Acute Exacerbation of Chronic Obstructive
Pulmonary Disease: A Randomized Trial of
Trimethoprim-Sulfamethoxazole versus Ciprofloxacin.
Clinical Infectious Diseases,Volume 51, Issue 2,
July 15, 2010, 143-149.
https://academic.oup.com/cid/article-abstract/51/2/143/301482?redirectedFrom=fulltext
Francois M Treatment of acute
otitis media (French). Arch Pediatr. 1995
January;2(1): 86-88.
https://pubmed.ncbi.nlm.nih.gov/7735433/
Werth B Trimethoprim and
Sulfamethoxazole. Merck manual Professional Version
(reviewed/revised May 2024).
https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-medications/trimethoprim-and-sulfamethoxazolePhthalylsulfathiazole
(a.k.a. sulfathalidine).
Creemers-Schild D Kroon F Kuijper
E de Boer M Treatment of Pneumocystis pneumonia with
intermediate-docent step-down to low-dose
trimethoprim-sulfamethoxazole: lessons from an
observational cohort study. Infection. 2015 October
15;44: 291-299.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4889633/
Masaki T Ishikawa K Fujino T
Koyamada R Kawai F Ota E Mori S Intermittent Versus
Daily Trimethoprim/Sulfamethoxazole Regimens for
Pneumocystis Pneumonia Prophylaxis: A Systematic
Review and Meta-Analysis.
https://academic.oup.com/ofid/article/11/9/ofae499/7747370
Eliakim-Raz N Hellerman M Yahav D
Cohen J Margalit I Fisher S Zusman O Shaked H
Bishara J Trimethoprim/sulfamethoxazole versus
vancomycin and the treatment of
healthcare/ventilator-associated MRSApneumonia: a
case control study. Journal of Antimicrobial
Chemotherapy, Volume 72, issue 3, March 2017,
882-887,
https://academic.oup.com/jac/article/72/3/882/2724583
Guideline for the Treatment of
Sinusitis, Otitis Media and Otitis Externa.
CarilionClinic Antimicrobial Stewardship. July 2021.
https://www.carilionclinic.org/sinusitis-otitis-guide
Beauduy C Winton L Sulfonamides,
Trimethoprim, & Quinolones Chapter 46 in Katzung's
Basic & Clinical Pharmacology (Vanderah TW, ed) 16e
McGraw Hill 2023.
Cadena J Nair S Henao-Martinez A
Jorgensen J Patterson J Sreeramoju P Dose of
Trimethoprim sulfamethoxazole To Treat Skin and Skin
structure Infections Caused by Methicillin-Resistant
Staphylococcus aureus. Antimicrob Agents Chemother.
2011 December;55(12): 5430-5432.
https://pmc.ncbi.nlm.nih.gov/articles/PMC3232808/
IDSA (Infectious Diseases Society
of America) Clinical Practice Guidelines for the
Diagnosis and Management of Skin and Tissue
Infections: 2014 Update by IDSA (last updated August
16, 2018).
https://www.idsociety.org/practice-guideline/skin-and-soft-tissue-infections/
Siddiqui A Koirala J Methicillin-Resistant
Staphylococcus aureus. StatPearls. National Library
of Medicine Bookshelf. (Last updated April 2, 2023).
https://www.ncbi.nlm.nih.gov/books/NBK482221/#
DuPont H Reves R Galindo E
Sullivan P Wood L Mendiola Treatment of traveler's
diarrhea with trimethoprim/sulfamethoxazole and with
trimethoprim alone. N Engl J Med. 1982 September
30;307(14): 841-844.
https://pubmed.ncbi.nlm.nih.gov/7050714/
Diemert D Prevention and
Self-Treatment of Travelers Diarrhea. Clin Microbiol
Rev. 2006 July;19(3): 583-594.
https://pmc.ncbi.nlm.nih.gov/articles/PMC1539099/
Sureshbabu J Shigella Infection
Medication (updated: March 3, 2023). Medscape
https://emedicine.medscape.com/article/968773-medication
Leibovici-Weissman Y Neuberger A
Bitterman R Sinclair D Salam M Paul M Antimicrobial
drugs for treating cholera. Cochrane Database Syst
Rev. 2014 to 19;2014(6):CD00825 (updated 23 March
2018).
https://pmc.ncbi.nlm.nih.gov/articles/PMC4468928/#
Li J Cui Z Qi M Zhang L Advances
in Cyclosporiasis Diagnosis and Therapeutic
Intervention. Front Cell Infect Microbiol. 2020
February 11;10:43.
https://pmc.ncbi.nlm.nih.gov/articles/PMC7026454/
Katia M Isosporiasis. EBSCO
(2025).
https://www.ebsco.com/research-starters/consumer-health/isosporiasis
Root H Daniels L Marx A Bartelt L
Lachiewicz A van Duin D Sulfonamides without
trimethoprim in the treatment of Nocardia
infections: A case report and literature review.
Transpl Infect Dis. 2021 February;23(1): e134452.
https://pubmed.ncbi.nlm.nih.gov/32869901/
Mazumder S Nocardiosis Treatment &
Management (Updated: December 30, 2024). Medscape.
https://emedicine.medscape.com/article/224123-treatment
Guidelines
for the Prevention and Treatment of Opportunistic
Infections in Adults and Adolescents with HIV.
Toxoplasmosis. (Reviewed January 8, 2025). Clinical
info HIV:
https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/toxoplasmosis
Sulfadoxine/pyrimethamine.
https://en.wikipedia.org/wiki/Sulfadoxine/pyrimethamine
Massere Y Braunack-Mayer L Miller
R Mohrle J Penny M A roadmap for understanding
sulfadoxine-pyrimethamine in malaria
chemoprevention. Parasitology. 2025 January
23;152(2): 133-142.
https://pmc.ncbi.nlm.nih.gov/articles/PMC12089447/
Malaria: Treatment. Mayo Clinic
February 9, 2023.
https://www.mayoclinic.org/diseases-conditions/malaria/diagnosis-treatment/drc-20351190
Bennett N Brucellosis Treatment &
Management. Medscape (updated: December 12, 2024).
https://emedicine.medscape.com/article/213430-treatment#d8
Silver Sulfasalazine. StatPearls.
National Library of Medicine Bookshelf. (Last
update: January 22, 2023).
https://www.ncbi.nlm.nih.gov/books/NBK556054/
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |