Medical Pharmacology Chapter 35 Antibacterial Drugs
Sulfonamides
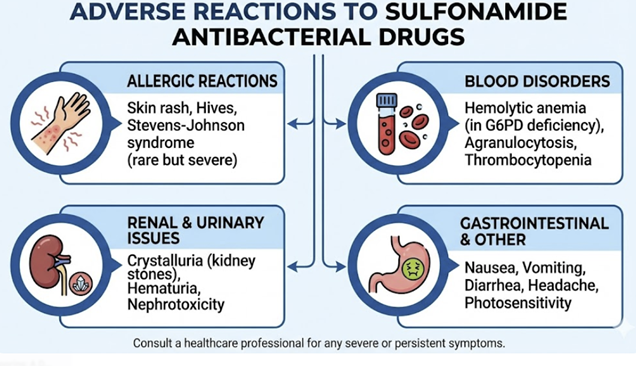 |
Hypersensitivity Reactions
Hypersensitivity reactions represent the most common category of adverse effects associated with sulfonamides.
Common Reactions
![]() The most
frequent manifestations are cutaneous, including morbilliform (maculopapular)
rashes and urticaria (hives).
The most
frequent manifestations are cutaneous, including morbilliform (maculopapular)
rashes and urticaria (hives).
Photosensitivity is also a well-documented effect, making it
essential to counsel patients on avoiding excessive sun exposure
and using sunscreen.
Mechanisms associated with phototoxicity may involve UV radiation causing drug molecule electrons to transition to an excited, chemically unstable state. The excited state participates in direct energy transferred oxygen creating a singlet oxygen which is the reactive oxygen species (ROS).
Oxidative stress associated with ROS formation as well as direct cellular damage caused by the free radical results in extreme sunburn--like reaction.4
Severe Cutaneous Adverse Reactions
These reactions are considered rare but extremely serious hypersensitivity reactions which constitutes medical emergencies.
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
|
SJS and TEN represent a continuum of the same disease process,
distinguished by the percentage of body surface area (BSA)
affected by epidermal detachment: <10% BSA for SJS, >30% BSA for
TEN, and 10-30% for SJS/TEN overlap.
This delayed-type hypersensitivity reaction may be
initiated when the drug or its reactive metabolites
trigger a massive, targeted attack on the body's own
skin cells (keratinocytes) by cytotoxic
T-lymphocytes (CD8+) and Natural Killer (NK) cells.
Resulting cell death is mediated by the release of
cytotoxic proteins, including granulysin (a key
mediator), Fas-Fas Ligand interactions, and perforin/granzyme.
Genetic predisposition plays a major role, with risk
being strongly associated with specific Human
Leukocyte Antigen (HLA) alleles, although the
specific alleles for sulfonamides are less
well-defined than for other drugs like carbamazepine
or allopurinol.
Clinical Presentation6,7
The reaction typically begins 1 to 3 weeks after starting the offending drug.
An initial prodrome of fever, malaise, and flu-like symptoms is followed by the abrupt onset of a painful, dusky red or purpuric rash that rapidly progresses to form flaccid bullae and extensive sheets of epidermal sloughing.
Severe, painful involvement of mucous membranes (oral, ocular, and genital) is a hallmark feature.
A positive Nikolsky sign (gentle lateral pressure causes the epidermis to shear off) is characteristic.
Management6,7
SJS/TEN is a medical emergency requiring immediate hospitalization, often in a burn unit or intensive care unit.
The single most critical intervention is the immediate withdrawal of the suspected causative drug.
Treatment is primarily supportive, focusing on wound care,
fluid and electrolyte management, nutritional support, and
pain control.
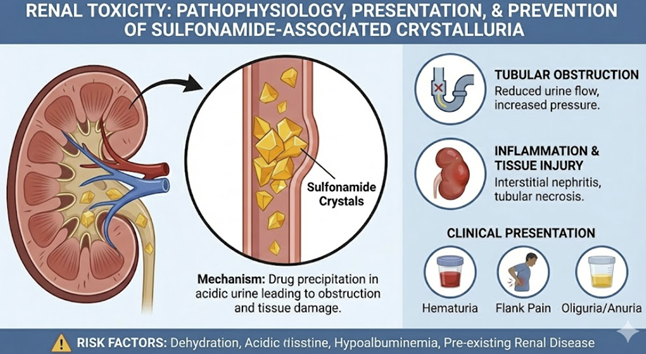 |
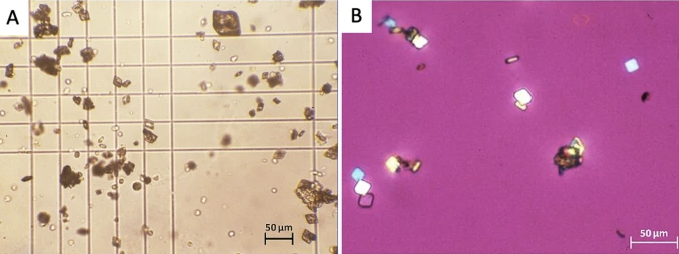
Low solubility can lead to their precipitation within the renal tubules and collecting ducts, forming crystals.
These crystals (classically described as "shocks of
wheat" for sulfadiazine) can aggregate to form
stones or sludge, causing tubular obstruction,
hematuria, and acute kidney injury.
This complication can be largely prevented by
ensuring the patient maintains adequate hydration to
produce a high urine output, and in some high-risk
situations, by alkalinizing the urine with sodium
bicarbonate to increase drug solubility.
 |
|
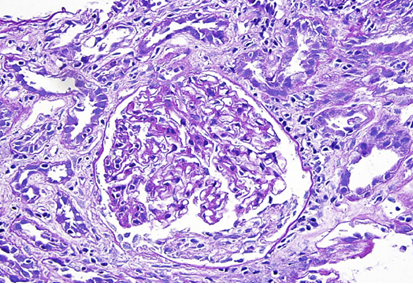
Acute Interstitial Nephritis (AIN)11,12,13
 |
|
This is a non-dose-related, idiosyncratic
hypersensitivity reaction that causes inflammation of
the kidney interstitium and tubules.
This hypersensitivity is a cell-mediated immune response, distinct from crystalluria.
The classic presentation includes fever, rash, and eosinophilia, accompanied by an acute decline in renal function.
The presence of eosinophils in the urine may be able marginally helpful diagnostic clue, though not always present.13
Hematologic Toxicity
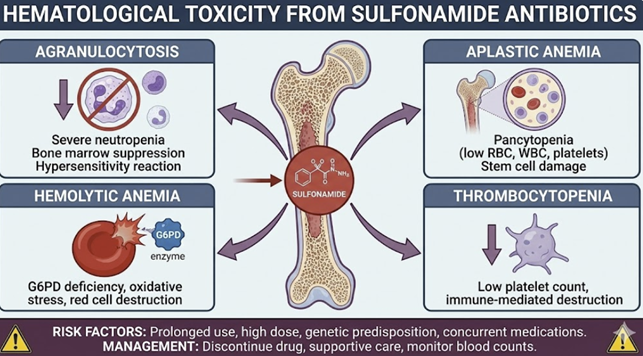 |
Agranulocytosis14
Agranulocytosis is a rare (<1%), unpredictable, but potentially fatal idiosyncratic reaction characterized by a profound decrease in the absolute neutrophil count to <0.5 x 109/L, which results in the patient being patient at high risk for severe infections.
Mechanism
This reaction is believed to be immune-mediated.
The current hypothesis suggests that reactive metabolites of the sulfonamide, formed within neutrophils or their bone marrow precursors by enzymes like myeloperoxidase, act as haptens.
These sulfonamides reactive metabolites, acting as haptens,
trigger an immune response that leads to mature neutrophils
destruction and/or suppression of their production.
A
strong association with certain HLA genotypes provides further
evidence for an immunogenetic basis.
Hemolytic Anemia15,16,17
Sulfonamides are oxidizing agents and can induce acute hemolysis in patients with an inherited deficiency of the enzyme glucose-6-phosphate dehydrogenase (G6PD).
In these individuals, red blood cells lack sufficient reducing power to protect themselves from oxidative stress, leading to their premature destruction.
Folate Deficiency18
While the affinity of trimethoprim for bacterial dihydrofolate reductase (DHFR) is many times greater than for human DHFR, prolonged use of TMP/SMX can still interfere with human folate metabolism.
Such interference can lead to megaloblastic anemia, leukopenia, and/or thrombocytopenia, particularly in patients with underlying risk factors such as malnutrition, alcoholism, or pregnancy.
The clinical importance of an interaction between trimethoprim-sulfamethoxazole and folic acid remains a topic for continued discussion, Noting the availability of folate in the diet.19
Hyperkalemia20
The trimethoprim component of TMP/SMX is structurally similar to the potassium-sparing diuretic amiloride.
Trimethoprim directly inhibits the epithelial sodium channel (ENaC) in the distal renal tubule, which reduces the excretion of potassium.
This effect may lead to clinically significant and potentially life-threatening hyperkalemia, especially in the elderly, patients with pre-existing renal insufficiency, and those taking concomitant medications that also raise potassium levels (e.g., ACE inhibitors, ARBs, spironolactone)
Drug-Drug Interactions
Metabolic Interactions (CYP2C9 Inhibition): Sulfamethoxazole is a moderate inhibitor of the cytochrome P450 isoenzyme CYP2C9, which is responsible for the metabolism of many commonly used drugs.21
Warfarin22
The warfarin-sulfamethoxazole (SMX) interaction may be, arguably, the most clinically important.
SMX significantly inhibits the metabolism of S-warfarin, the more potent enantiomer of the anticoagulant.
This inhibition leads to a rapid and often unpredictable increase in the International Normalized Ratio (INR) and places the patient at a very high risk of serious or fatal bleeding.
![]() If the combination cannot be avoided, extremely
close monitoring of the INR (potentially daily)
and proactive dose reduction of warfarin is
required.
If the combination cannot be avoided, extremely
close monitoring of the INR (potentially daily)
and proactive dose reduction of warfarin is
required.
Sulfonylureas20,23,24
SMX inhibits the metabolism of oral hypoglycemic agents like glyburide and glipizide.
Inhibition of metabolism of these oral hypoglycemic drugs potentiates their glucose-lowering effect and can precipitate severe, prolonged hypoglycemia.
![]() Patients
with diabetes taking these agents require frequent blood
glucose monitoring and may need dose adjustments.
Patients
with diabetes taking these agents require frequent blood
glucose monitoring and may need dose adjustments.
Phenytoin20,25
Sulfamethoxazole inhibits the metabolism of phenytoin, which can lead to elevated serum levels and an increased risk of dose-related toxicity (e.g., nystagmus, ataxia)
Protein-Binding and Renal Excretion Interactions
Methotrexate26,27
The combination of TMP/SMX and methotrexate is particularly hazardous and should generally be avoided.
The interaction is multi-faceted: SMX can displace methotrexate from its binding sites on plasma albumin, increasing the free, active fraction of the drug, while TMP competes with methotrexate for active tubular secretion in the kidneys, decreasing its clearance.
![]() Both
mechanisms lead to elevated methotrexate levels and a profound
risk of toxicity, including severe myelosuppression, mucositis,
and renal failure.
Both
mechanisms lead to elevated methotrexate levels and a profound
risk of toxicity, including severe myelosuppression, mucositis,
and renal failure.
The trimethoprim component has a potassium-sparing effect.
When used concurrently with other drugs that raise potassium levels, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), there is an additive risk of developing hyperkalemia.
Digoxin30
Sulfonamides have been reported to increase serum digoxin levels, potentially leading to toxicity.
July, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |