Medical Pharmacology Chapter 35 Antibacterial Drugs
 |
 |
 |
 |
 |
 |
Quinolone antibiotics (especially the fluoroquinolones) are a class of synthetic, broad-spectrum antibacterials originally derived from nalidixic acid.
These drugs have become important agents in human medicine due to their potent bactericidal activity, excellent oral bioavailability, and broad tissue penetration.1
|
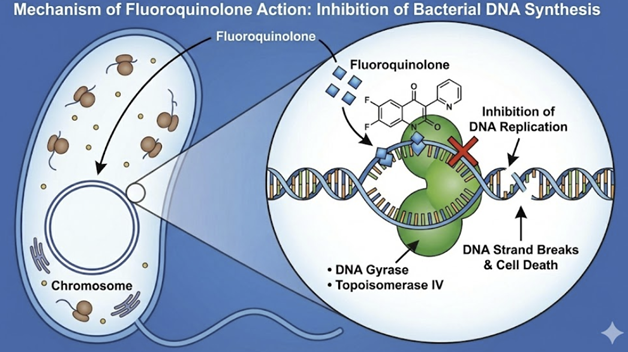
Quinolones occupy a distinctive position in antimicrobial therapy through their direct inhibition of bacterial DNA synthesis.2,3
Unlike other antibiotic classes that target cell wall synthesis or protein production, quinolones specifically target two essential bacterial enzymes: DNA gyrase and topoisomerase IV.3,4,5
DNA-Gyrase and topoisomerase IV our central for bacterial DNA replication transcription which makes of these enzymes important therapeutic targets.3,6
Quinolones (including fluoroquinolones) are bactericidal agents targeting DNA replication.7
Bacterial DNA synthesis is inhibited by stabilizing DNA complexes of DNA with two essential bacterial enzymes: DNA gyrase (also known as topoisomerase II) and topoisomerase IV.1
At the molecular level, quinolones “trap” gyrase or topoisomerase IV on DNA, forming a ternary complex that halts replication forks and generates lethal double-strand breaks when the blocked replication machinery collides with these complexes.9,10
![]() By
binding to these enzymes, quinolones promote cleavage of
bacterial DNA and prevent the resealing of the DNA strands,
leading to fragmentation of the chromosome and rapid cell death.
By
binding to these enzymes, quinolones promote cleavage of
bacterial DNA and prevent the resealing of the DNA strands,
leading to fragmentation of the chromosome and rapid cell death.
Generally, Gram-negative antibacterial activity correlates with inhibition of DNA gyrase, whereas Gram-positive activity correlates with inhibition of topoisomerase IV.1
Quinolones exhibit concentration-dependent killing, meaning their efficacy improves as drug concentration increases relative to the organism’s MIC (minimum inhibitory concentration) until the optimum bactericidal concentration is reached, after that the bactericidal activity declines.8
Peak bactericidal effect is typically observed when concentrations reach ~30× MIC.
Quinolones also produce a post-antibiotic effect which consists of continued bacterial growth suppression even after drug levels fall below the MIC, lasting roughly 1–2 hours.
These pharmacodynamic properties allow quinolones to be dosed once or twice daily for most infections.
|
|
"Structure bacterial DNA-Gyrase complex
with DNA and two ciprofloxacin molecules (green)"14
|
|
|
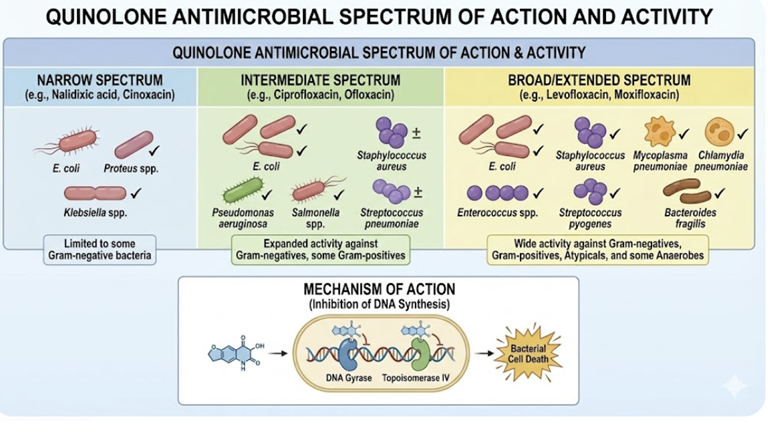
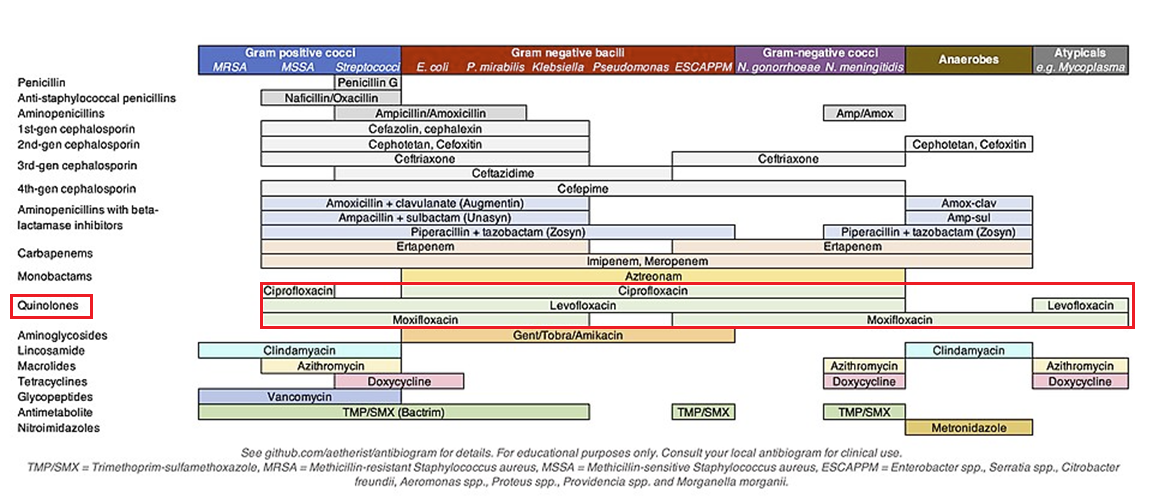
Antimicrobial Spectrum and Activity
 |
 |
|
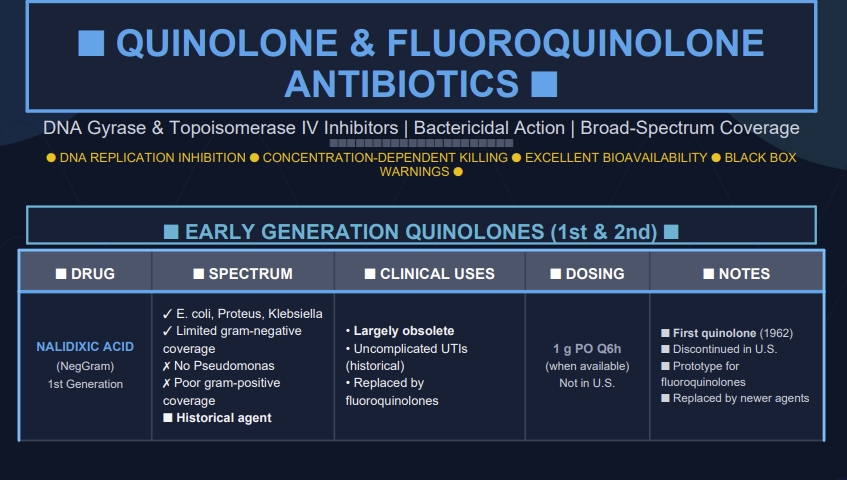
First-generation quinolones
Early quinolones (e.g. nalidixic acid) were mainly active against Enterobacteriaceae and other Gram-negative rods, with minimal systemic distribution, limiting them to urinary tract infections.1
First-generation drugs included:
Nalidixic acid
Cinoxacin
Flumequine,
Oxolinic acid,
Piromidic acid
Pipemidic acid and
Rosoxacin20
First Generation agents primarily cover Gram-negative bacilli such as E. coli, Klebsiella species and Proteus species in uncomplicated UTIs, and are not used for systemic infections.14
Three drugs were administered orally and exhibited low serum in tissue levels.
First-generation agents required frequent daily administration (four times daily) as well as an elevated tendency to select for resistant Gram-negative bacilli. They also exhibited limited or poor activity against Gram-positive bacteria as well as inducing photosensitivity reactions in patients. First generation quinolones had the potential to induce convulsions in patients with seizure disorders.19
They also exhibited narrow Gram-negative bacterial coverage.1
Second-generation quinolones
Second generation agents including fluorine atom in their structure.
This modification resulted in improved antimicrobial activity against Gram-negative bacteria and extends it to some Gram-positive bacteria.14
Second generation fluoroquinolones targeted Gram-negative organisms including Pseudomonas aeruginosa as well as some Gram-positive bacteria extending to Staphylococcus aureus but not to Streptococcus pneumoniae as well as to some atypical pathogens.21
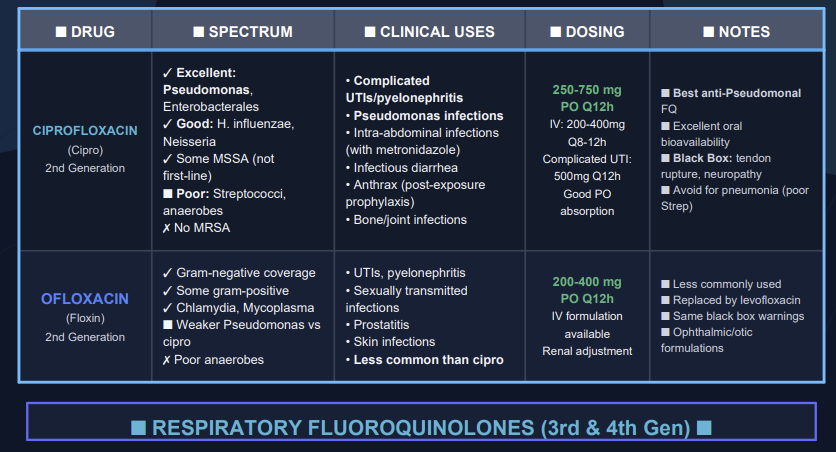
Second generation agents include ciprofloxacin, enoxacin, lomefloxacin, ofloxacin, fleroxacin, ofloxacin and rufloxacin.
Of these, ciprofloxacin is the most potent fluorquinolone against P.aeruginosa.
Some second-generation quinolones may be useful in treating lower respiratory tract infections and acute sinusitis; however, S. pneumoniae is often resistant to these drugs.
Accordingly, second-generation quinolones would not be drugs of first choice for lower respiratory tract infections or acute sinusitis. Among the second-generation drugs, ofloxacin exhibits highest activity against Chlamydia trachomatis.
The most widely used second-generation quinolones are likely ciprofloxacin and ofloxacin due to both oral and IV formulation as well as extensive FDA-labeled indication for use.21
![]() Clinical
use of second-generation agents include both uncomplicated and
complicated urinary tract infections as well as pyelonephritis.
Clinical
use of second-generation agents include both uncomplicated and
complicated urinary tract infections as well as pyelonephritis.
Other uses include treating sexually transmitted diseases, skin and soft tissue infections, and prostatitis.21
So, with respect to Legionella pneumophila, for example, ciprofloxacin exhibits good activity.23
Good activity by ciprofloxacin is also noted against Pseudomonas aeruginosa as well.
So the main inadequacy is lack of coverage again Streptococcus pneumoniae, meaning that ciprofloxacin administration would be inappropriate for management of community-acquired pneumonia.
To summarize then, ciprofloxacin and ofloxacin would be expected effective in managing:
(1) Urinary tract infections caused by nonresistant bacteria,
(2) Respiratory tract infections caused by susceptible Gram-negative bacteria,
(3) Skin and soft-tissue infections and
(4) Osteomyelitis [ciprofloxacin only].23
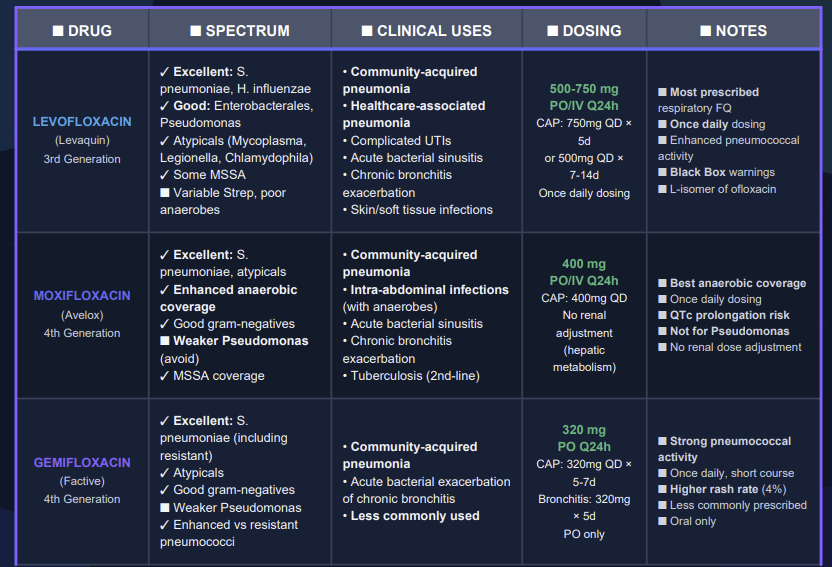
Third-generation quinolones
Third-generation quinolones have properties similar to the second generation but add expanded Gram-positive coverage including penicillin-sensitive and penicillin-resistance Staphylococcus pneumoniae (S. pneumoniae).
![]() The
expansion of sensitive organisms targeted by
third-generation quinolones, now including Gram-positive
coverage of Streptococcus pneumoniae, now allowed
quinolones to be a reliable option for empirical
treatment of community acquired pneumonia.23
The
expansion of sensitive organisms targeted by
third-generation quinolones, now including Gram-positive
coverage of Streptococcus pneumoniae, now allowed
quinolones to be a reliable option for empirical
treatment of community acquired pneumonia.23
In addition there is expanded coverage of atypical pathogens, such as Mycoplasma pneumoniae and Chlamydia pneumoniae.21
Notable clinical uses include community-acquired pneumonia as well as acute worsening of chronic bronchitis.21
Examples of third-generation quinolones include balofloxacin, grepafloxacin, levofloxacin, pazufloxacin, temofloxacin.
To summarize then, third-generation quinolones would be expected effective in managing:
(1) Community-acquired pneumonia
(2) Bacterial exacerbations of acute bronchitis
(3) Urinary tract infections and
(4) Skin infections.23
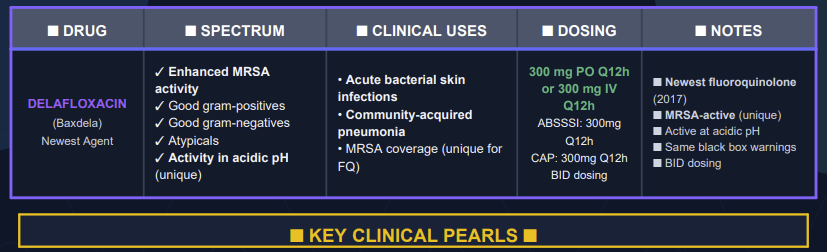
Fourth-generation quinolones22
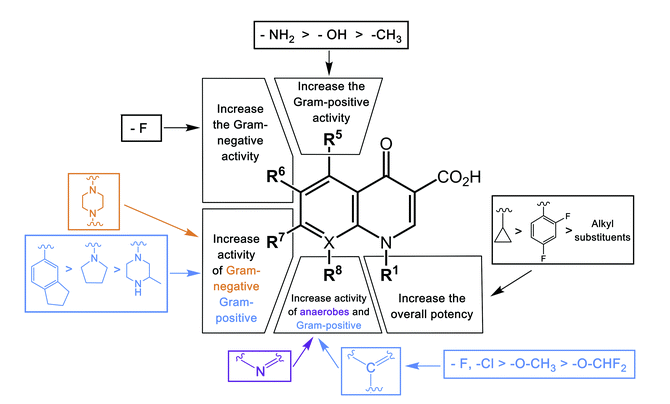
The fourth generation quinolones incorporated number of structural side chain modifications that:
Reduce quinolone efflux out of bacterial cells
Increase serum half-life
Increase potency against Gram-positive bacteria and
Add important anaerobic coverage.
Notable is a C-8 methoxy group that enhances potency while reducing toxicity.
![]() An
example of the fourth-generation agent, moxifloxacin, exhibits
enhanced an equal affinity for topoisomerase II (DNA-Gyrase) and
topoisomerase IV.
An
example of the fourth-generation agent, moxifloxacin, exhibits
enhanced an equal affinity for topoisomerase II (DNA-Gyrase) and
topoisomerase IV.
These enhancements make moxifloxacin potent against a broader range of pathogen while being less likely to be susceptible to mutant which exhibits they single resistance target to either one of the two topoisomerases.22
Examples of fourth-generation fluoroquinolones include: clinafloxacin, moxifloxacin sitafloxacin, prulifloxacin, besifloxacin, delafloxacin.
To summarize then, fourth-generation quinolones would be expected effective in managing:
(1) Nosocomial pneumonia,
(2) Intra-abdominal infections,
(3) Serious penicillin-or cephalosporin-resistant Streptococcus pneumoniae infections.23
 |
|
July, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |