Medical Pharmacology Chapter 35 Antibacterial Drugs
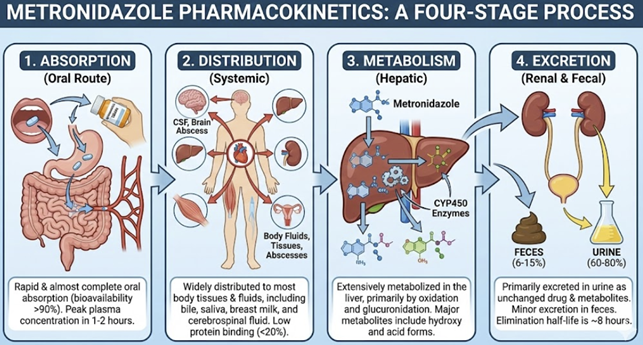 |
Absorption
Metronidazole is efficiently absorbed after oral administration, with about 80% bioavailability.1
Peak plasma concentrations are reached~1–2 hours following they dose.
Oral absorption is not significantly reduced by food (though food may delay the peak slightly).1
Because of its high oral bioavailability, oral and IV dosing achieve similar systemic levels, allowing outpatient therapy for serious infections once the patient can tolerate oral intake.2
Distribution
Metronidazole distributes widely throughout body tissues and fluids. It has low protein binding (~20% bound)1, leaving most of the drug free to penetrate tissues.
Metronidazole is notably excellent at penetrating anaerobic abscesses and body cavities.
Metronidizole achieves therapeutic concentrations in the central nervous system and cerebrospinal fluid (about 43–100% of plasma levels), making it effective for brain abscess and anaerobic meningitis.4
Metronidizole also crosses the placenta and is excreted into breast milk.
Because it distributes into amniotic fluid and the fetus, metronidazole has been scrutinized in pregnancy.5
Metronidazole also diffuses into saliva, bile, seminal fluid, and vaginal secretions, reflecting its extensive distribution.
Metabolism
Metronidazole is primarily and extensively metabolized by the liver.6
Metronidizole is metabolized by the liver to 5 metabolites.
The hydroxy metabolite exhibits biological activity of 30%-65% with the longer elimination half-life compared to the parent drug.
About 30–60% of a dose is oxidized by hepatic enzymes7 (including CYP2A6 and others) to metabolites such as 2-hydroxymetronidazole and a metronidazole acid derivative.8
Metronidazole undergoes glucuronide conjugation.9
Hepatic metabolism means that severe liver impairment can result in much higher plasma drug levels10 and a prolonged metronidazole’s half-life.11
In patients with advanced liver disease (Child-Pugh class C), the elimination half-life can double, and dose reduction (by ~50%) is recommended.12
Mild to moderate hepatic impairment does not usually require adjustment, though close monitoring is advised.12
Excretion
Both metronidazole and its metabolites are excreted mainly in the urine (≈77%), with a smaller portion via feces (~14%).1,9
Unmetabolized metronidazole accounts for about 20% of the excreted drug in urine.9
Because of the colored metabolites, patients often notice dark or reddish-brown urine while on metronidazole.13,14
Half-life
In healthy adults, the elimination half-life of metronidazole is about 8 hours.1 The half-life is significantly prolonged in neonates and young infants (e.g. ~23 hours in a 2-month-old, and up to 100 hours in premature neonates.)1
August, 2025
|
|
This Web-based pharmacology and disease-based integrated teaching site is based on reference materials, that are believed reliable and consistent with standards accepted at the time of development. Possibility of human error and on-going research and development in medical sciences do not allow assurance that the information contained herein is in every respect accurate or complete. Users should confirm the information contained herein with other sources. This site should only be considered as a teaching aid for undergraduate and graduate biomedical education and is intended only as a teaching site. Information contained here should not be used for patient management and should not be used as a substitute for consultation with practicing medical professionals. Users of this website should check the product information sheet included in the package of any drug they plan to administer to be certain that the information contained in this site is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. Advertisements that appear on this site are not reviewed for content accuracy and it is the responsibility of users of this website to make individual assessments concerning this information. Medical or other information thus obtained should not be used as a substitute for consultation with practicing medical or scientific or other professionals. |